|
|
|
|
Ozone Discourses: Science and Politics in Global Environmental Cooperation , by Karen T. Litfin
| The measures agreed here are the strongest package of global environmental law ever enacted. The question remains, however: is this enough? We are in the hands of scientists. From them we know that the answer is "No." |
| -Mostafa Tolba, Copenhagen, 1992 |
Perhaps the greatest innovation in the Montreal Protocol is the institution of periodic treaty reviews based on new knowledge. Although the treaty was hailed as a diplomatic milestone for its precautionary approach to a global environmental problem, the nonlinear relationship between CFCs and ozone depletion indicated that far more drastic measures would be necessary. Within a matter of months after Montreal, a scientific consensus had emerged on three vital issues: that the Antarctic ozone hole was caused by CFCs, that ozone losses were occurring globally, and that the stratospheric chemistry above the Arctic was highly perturbed.
Superficially, it appears that, once CFCs were clearly identified as the culprits, the debates were no longer formulated in terms of science but reduced to the more traditional dynamics of bargaining and political compromise. To be sure, the emerging scientific consensus was mirrored in a new symmetry between the positions of the USA and the EC. In comparison to the Montreal Protocol negotiations, debates about scientific knowledge were quite scarce in the post-Montreal period. A consensus on these issues had been forged, thereby facilitating, though not necessitating, the emergence of a political consensus. The major source of contention soon became the issue of assisting developing nations to obtain CFC substitutes, chemicals that would cost three to five times as much as the ozone-depleting CFCs. Yet the developing countries did not contest the science; nor did they frame the issue in scientific terms. Rather, they expressed their concerns in terms of economic equity and national sovereignty.
While there is some merit in this interpretation of events, it fails to recognize the ways in which scientific and technical discourse continued to be a driving force behind the treaty revision process. And, in downplaying the role of knowledge, this view inevitably overlooks the extent to which policy was shaped by specific modes of framing and interpreting the available knowledge. Moreover, on at least one issue - control measures for methyl bromide - there has been substantial scientific disagreement between North and South. Throughout the amendment process, as in the earlier negotiations, science did not simply provide a set of objective and value-free facts from which a policy consensus was forged. Rather, distinctive ways of presenting and interpreting information were instrumental in shaping policy outcomes.
The interplay between science and politics has reemerged during the treaty revision process in many of the same guises discussed in the preceding chapter. One particular method of framing the available scientific knowledge, the chlorine-loading approach, dominated the treaty revision deliberation. As before, this interpretive mode gained salience from contextual factors gleaned from observational data, but it was not mandated by the data. That schematic approach to the data, elaborated after the Montreal Protocol by knowledge brokers from the EPA and NASA, shaped the revised control measures adopted in 1990 and 1992.
Further evidence of the multidimensional relationship between science and politics, one that was touched on in the last chapter, is the problem of substitute availability. Superficially, this seems to be a technical matter involving chemical research. Initially, industry raised strong objections to the 50 percent reductions in CFC emissions called for in the Montreal Protocol, claiming that substitutes would not be available to meet the control schedule. Yet, within three years, the CFC industry was largely supportive of a full phaseout within the same time frame. The astonishing speed of technological development has been, as much as the new scientific findings of increased ozone depletion, a driving force behind the treaty amendment process. The availability of substitutes enabled policymakers to adopt far more stringent controls than they otherwise might have. But that availability, I will argue, was dictated by psychological and market forces, not by chemistry or technology, and these forces were in turn guided by scientific and policy discourse.
To attribute the post-Montreal discursive shift to an epistemic community, as Peter Haas does (1992a:215–18), is to truncate the story. While knowledge brokers were important players, their central role was in framing the science rather than in promoting specific policies. The epistemic communities approach misses this fundamental dimension of interpretation. In his brief discussion of the treaty revision process, Haas actually refers to environmental nongovernmental organizations rather than to an epistemic community in accounting for words and outcomes. While a kind of ozone "club" of negotiators and experts had developed over the years, the bonding principles of that group were not so much epistemic as it was political and personal (interview with Eileen Claussen).
The post-Montreal events can be read as an extension of the precautionary discourse launched with the Vienna Convention and formalized in the Montreal Protocol. And yet, once unprecedented ozone losses were being measured and once the Antarctic ozone hole was decisively linked to human sources, the control measures were no longer truly precautionary. Action did not precede evidence of environmental degradation but instead responded to it in order to prevent things from going from bad to worse. The question really became how to save the ozone layer and, in the long run, how to repair the damage already done. Hence, it is more accurate to speak of a shift toward a dominant conservation discourse, which was articulated in London in 1990 and Copenhagen in 1992.
Immediate Responses
Even before the ink on the Montreal Protocol had dried, scientists were uncovering new data that would dispel many uncertainties. Scientists on the second Antarctic expedition, announcing their preliminary results just two weeks after the treaty was signed, concluded that CFCs had caused the hole (Washington Post 1987h; Nature 1987a). Immediately, many calls to amend the treaty were heard (Nature 1987a; Washington Post 1987i).
The Montreal Protocol was roundly praised by the press (see Newsweek 1987; The Washington Post 1987g). Yet the disturbing news from Antarctica suggested that the treaty may have done too little, too late. For the first time since the 1970s, environmentalists began to mobilize on a large scale at the grassroots level to save the ozone layer. The easiest targets were aerosols and foam packaging, for which alternatives were readily available (Shea 1988:35). In the United States, the city of Berkeley promptly banned fast-food packaging made with CFCs, and activists convinced McDonald's to abandon its use of styrofoam containers made with CFCs (Washington Post 1987). Soon afterward, environmental groups, working with the EPA, negotiated an agreement with U.S. styrofoam manufacturers to stop using CFC-11 and CFC-12 by the end of 1988. In Canada, a major grocery chain removed CFC-based packaging from its shelves. The European Environmental Bureau (EEB) and the European Bureau of Consumers' Unions (BEUC) formed a powerful alliance, calling for an 85 percent reduction in CFC use and demanding that industry implement voluntary reductions beyond those in the protocol or else face a major boycott (Jachtenfuchs 1990:268). In Britain, Friends of the Earth (FOE) announced a boycott of aerosol products containing CFCs, causing ICI to pledge a halt to the use of CFCs in aerosols by 1990. Prince Charles joined the boycott, banning the products from his household (FOE 1988). Initially, environmentalists thought that the ozone problem was a clear-cut consumer issue. Yet once CFCs were eliminated in aerosols and food packaging, there was little that could be done through boycotts; consumers were unlikely to stop buying refrigerators and cars (interview with Liz Cook).
European industry, especially in Britain and France, was critical of the agreement, continuing to question the CFC–ozone depletion link (Environmental Data Services 1988). While expressing considerable ambivalence, U.S. industry was a bit more receptive. The Alliance for a Responsible CFC Policy praised the treaty as "an unprecedented step to protect the global environment" while persisting in its claim that "current use of the compounds presents no significant risks to health or the environment" (1987:I.8–9).
Despite industry's reluctance, the treaty and the ensuing calls for revisions propelled it into an unprecedented race to find chemical substitutes. Recognizing that marketability for CFC substitutes could be hastened through cooperation on toxicological and environmental testing, fifteen CFC producers from nine countries joined together in an innovative effort to expedite research on new chemicals. In December 1987, the Programme for Alternative Fluorocarbon Toxicity Testing (PAFT), based at ICI in Britain, was launched to address the toxicology of two compounds: HCFC-123 and HFC-134a, potential substitutes for CFC-11 and CFC-12, respectively. Within a year, twelve chemical producers formed the Alternative Fluorocarbons Environmental Acceptability Study (AFEAS), based in Washington, D.C., to assess the environmental risks of potential CFC substitutes (AFEAS/PAFT 1991). Since then research results have been pooled among the member companies and forwarded to the appropriate regulatory agencies to speed the approval process.
Industry's interest in CFC replacements was evident at a Substitutes and Alternatives trade fair cosponsored by the EPA and Environment Canada in January 1988. Several hundred CFC users and producers packed the workshop to the point of overflow. The Montreal Protocol had become a marketing opportunity for new products. Alternative foam-blowing agents like methylene chloride, pentane, and carbon dioxide, were discussed, as were hydrocarbon aerosol propellants, used widely in the USA since the 1978 aerosol ban. Petroferm, a small Florida-based company, unveiled a substitute solvent for CFC-113 made from chemicals found in citrus fruit rinds and tested successfully by AT&T (New York Times 1988b). An independent study presented at the workshop found that this new compound alone could replace as much as one-half of total projected CFC-113 use in the U.S. electronics industry. Soon after the workshop, Du Pont announced plans to build a new plant in Texas to manufacture HFC-134a, a chlorine-free refrigerant that would sell for about seven times the price of CFC-12 (Wall Street Journal 1988b; Washington Post 1988b). Du Pont and several other CFC producers began actively to promote the partially halogenated HCFC-22. 1
Not only did the Montreal Protocol herald markets for new chemicals, it spurred the search for alternative practices and technologies that could reduce the need for CFCs. Even before 1987, new product designs, like side vent windows in cars and solar ventilation systems for indoor heating, were being promoted by some environmentalists (Miller and Mintzer 1986). The level of interest increased greatly after Montreal. Halon emissions could be reduced significantly by not discharging during testing. New refrigerator designs could use helium or ammonia refrigerants instead of CFCs (Shea 1988:30–31). Despite their objections to regulation, both CFC users and producers were determined to make the best of the situation.
Industry was not the only ambivalent actor; some developing countries were also skeptical of the treaty. A few, most notably Mexico, the first nation to ratify the protocol, as well as Egypt, Venezuela, Kenya, and Indonesia, were quite supportive. Malaysia, however, was an outspoken critic of the treaty, claiming that the "inequitable" treaty was equivalent to "trade war by environmental decree" (Jaafar 1990; cited in Benedick 1991:100). While Malaysia's actions were unlikely to damage the ozone layer substantially, its language was adopted by other developing countries. In particular, India and China, together accounting for 40 percent of the world's population, registered strong reservations to the treaty. No sooner had the Montreal Protocol been negotiated than the stage was set for a confrontation between North and South.
The EC was divided on how to implement the protocol. At a December 1987 meeting, the United Kingdom proposed that the EC as a unit, rather than individual countries, should meet the treaty's guidelines. The FRG, the Netherlands, and Denmark, adopting their familiar environmentalist stance, advocated going beyond the protocol toward an eventual CFC ban (European Commission 1987). Recognizing that the easiest way to reduce CFC emissions quickly was to cut their use in aerosols, as the United States had done a decade earlier, European companies began to phase out CFCs as propellants (Benedick 1991:107). 2 The Netherlands acted most quickly, banning CFCs in aerosols in response to a national campaign organized by environmental groups (FOE 1988).
Amid growing concern, UNEP's executive director, Mostafa Tolba, convened a meeting of representatives from governments, industry, and nongovernmental organizations (NGOs) in January 1988 to discuss implementation of the Montreal Protocol. Despite the increased level of activity in industry and environmental groups, no nation had yet ratified the treaty. In order for the treaty to enter into force by the target date of January 1, 1989, ratification was required by nations or regional economic integration organizations representing at least two-thirds of 1986 CFC consumption (article 16 of the Montreal Protocol, UNEP 1987a). The January meeting generated an implementation timetable, scheduling three important meetings for October 1988 in The Hague: a scientific symposium to review new ozone research, a meeting of legal and technical experts to set the agenda for the First Meeting of the Parties, and a technical workshop for industry on substitutes and alternative technologies. And, recognizing that the treaty revision process should progress quickly, the group also decided to move up two critical dates: the First Meeting of the Parties was rescheduled from November to May 1989 and the scientific, environmental, economic, and technological assessments were to be completed in 1989 rather than in 1990 (Benedick 1991:109).
Thus, within four months of Montreal, preparations were under way for a revised treaty. Article 6, mandating assessment and review of the control measures beginning in 1990 and at least every four years thereafter, may have been the most important element of the treaty. A combination of new scientific evidence, renewed and expanded activism on the part of environmental groups, and industry's energetic pursuit of new technologies was already creating a discursive shift in support of more decisive action to control ozone depleting chemicals.
New Science and the Discourse of Damage Limitation
On March 15, 1988, the Ozone Trends Panel, established by NASA in collaboration with UNEP, the WMO, and two other U.S. agencies to reanalyze nearly all satellite and ground-based measurements of ozone, released the executive summary of its report. The panel, formed in October 1986 and involving over a hundred scientists from ten countries, was primarily a response to Donald Heath's controversial satellite data. That data had shown annual global ozone losses of 1 percent since 1979 (interviews with Ralph Cicerone and Robert Watson). The panel concluded that actual losses were about half what Heath had measured, with greater decreases in the upper stratosphere ranging from 3 to 9 percent (NASA 1988:1–4). Most important for galvanizing political concern was the finding that significant ozone depletion had already occurred over heavily populated areas in the Northern Hemisphere, ranging from 1.7 to 3.0 percent, with far greater losses in the winter months. The modeled predictions for these latitudes were only 0.5 percent, casting even more doubt on the reliability of the computer models. The Ozone Trends Panel also reported the findings of the second Antarctic expedition. Large amounts of chlorine gas were found in the stratosphere during the formation of the ozone hole, indicating that CFCs and other anthropogenic sources of chlorine and bromine were depleting the Antarctic ozone layer. Moreover, ozone losses were not limited to the Antarctic spring, but were observed year-round (NASA 1988:19).
Many observers, both scientists and laypersons, were troubled by the fact that the full report would not be available for several months, thereby leaving the basis for the executive summary's conclusions unavailable for public scrutiny. One science writer, describing a host of complex factors involved in stratospheric chemistry and data calibration, noted that, "although many problems with the data have no doubt been addressed in some way, the science would be best served if the methods used were published, as is presumably the intent in the full report" (Trenberth 1988:26). With the future of international controls on ozone-depleting chemicals at issue, the timely release of the full report was important not only from a scientific perspective but also from environmental, political, and economic standpoints.
Yet it was nearly three years before the full report was finally published, causing some people, particularly those representing affected industries, to claim that the delay was caused by squabbling among the scientists (interview with Kevin Fay). That claim, however, was laid to rest in the minds of most observers when Allied-Signal commissioned an independent reanalysis of the data, released in August 1988, that substantiated the findings of the Ozone Trends Panel. Apparently, the tardiness of NASA's final report was actually the consequence of a series of administrative blunders. In the end, NASA's budgetary allocations for document publication having been spent, the industry consortium AFEAS contributed the funds needed to print the document (interview with Robert Watson). Although the report itself established an unprecedented consensus implicating CFCs in ozone depletion, the delay in publication furnished a handful of skeptics with further grounds for their position. To some extent, the credibility of scientific information became hostage to bureaucratic procedures.
Nonetheless, the executive summary of the Ozone Trends Panel Report, essentially performing a knowledge brokerage function, was generally accepted on the basis of the authority of the scientists involved and their sponsoring agencies. The document prompted more calls for a strengthened Montreal Protocol. Those asking for the revision noted that the treaty's emission targets still allowed atmospheric concentrations of CFCs to double from their 1986 levels (interviews with John Hoffman and David Doniger). On the basis of the new scientific findings, Sweden became the first country to ban CFCs, passing legislation in June 1988 to implement specific plans for a full phaseout. Although Sweden used only 1 percent of the world's CFCs, its bold action showed that a global phaseout was not inconceivable (Shea 1988:34).
But nowhere was the reaction swifter than at Du Pont, the world's leading CFC producer. Less than a week after the report's release, the company announced that it would halt all production of fully halogenated CFCs as soon as possible (Glas 1989:150). The announcement came as a surprise; only three weeks before, Du Pont's chief executive officer had written to three U.S. senators that "scientific evidence does not point to the need for dramatic CFC emission reductions." He claimed that reductions beyond those mandated by the Montreal Protocol would be "both unwarranted and counterproductive" (cited in Reinhardt 1989:15). Yet after hearing about the panel's findings on the evening news, Joseph Glas, director of Du Pont's Freon Division, met the next day with Environmental Manager Joseph Steed, and the division's top ozone scientist, Robert McFarland. McFarland, the only industry representative on the Ozone Trends Panel, had read the entire draft report but had been "sworn to secrecy" until the executive summary was released (interview with Robert McFarland). At the meeting, "the business end of things was never even discussed" (interview with Joseph Steed). On the basis of McFarland's corroboration of the panel's findings, Glas recommended to Du Pont's board of directors that the company stop manufacturing all chemicals regulated by the Montreal Protocol by 1999.
The Du Pont decision, while apparently demonstrating the persuasive power of consensual scientific knowledge, was not so simple. Contending interpretations of Du Pont's actions suggest once more that scientific facts alone rarely drive policy decisions. Rather, science, framed in terms of interests and perceptions, interacts with existing discourses to produce a new discursive milieu. The timing of Du Pont's decision, just after the Ozone Trends Panel released its findings and only weeks after the firm's chairman had insisted that further CFC reductions were unnecessary, supports the view that Du Pont was motivated primarily by the new scientific findings. While Du Pont was the leading researcher on CFC substitutes, the company's supporters argue that the decision to forfeit existing markets - amounting to $600 million in revenues for 1987 - for the sake of a vague future could not have been motivated solely by the quest for profits. Proponents of this interpretation also emphasize that Du Pont's corporate culture is very science-oriented, that "Du Pont's name has historically been synonymous with science" (Reinhardt 1989:9).
The most cynical explanation for Du Pont's decision comes, not surprisingly, from environmentalists, who maintain that the company was motivated primarily by the bottom line. FOE published a report in which they point out that Du Pont's CFC business was declining and that the Freon Division's operating profits were far below those of the company's other divisions. 3 The prices of CFC replacements would be several times those of existing compounds, and existing plants for manufacturing CFC-11 and CFC-12 could be retrofitted to produce HCFC-22. Du Pont also strategically renamed the partially halogenated chlorofluorocarbons, formerly called CFCs along with those regulated by the Montreal Protocol, "HCFCs" (FOE 1991d:43). Clearly, the Montreal Protocol furnished a marketing opportunity for any company that could take the lead in producing substitutes. Ironically, the account offered by U.S. environmentalists echoes the reaction of some representatives from the European CFC industry, who labeled the ozone treaty "the Du Pont Protocol" (interview with Mike Harris).
A middle position is that Du Pont's decision grew out of a combination of respect for scientific knowledge, practical concern for profitability, and corporate responsibility. Du Pont, a huge company with only 2 percent of its profits generated by CFC production, was unwilling to risk its public reputation for the sake of one family of dangerous compounds (interview with Stephen Seidel). 4 As Joe Steed declared, "Since we are two hundred years old, we tend to take a longer view. We couldn't let the whole company get a bad name just because of those chemicals. . . . I've found that the big corporations actually tend to be the most responsible, contrary to what most people to think. We have to be more concerned with our reputations" (interview). This interpretation of Du Pont's action combines the motivation of economic self-interest with a sense of corporate responsibility. It also suggests that the company's decision was inspired by considerations that went far beyond the scientific data.
Even more remarkable than Du Pont's action was the reversal in the British position. Prime Minister Margaret Thatcher was deeply distrustful of the Ozone Trends Panel report. The panel was international and included British scientists, but Thatcher was suspicious of NASA's leadership role in it. She requested that the British Stratospheric Ozone Research Group (SORG) assess the issue. SORG published a summary of its findings in June 1988 and the full report in October, corroborating the NASA report and supporting a phaseout of CFCs and halons. Scientists, including the head of Britain's environmental ministry, Crispin Tickell, appealed to Thatcher on the basis of her training as a chemist. She was also under some political pressure, the House of Lords having just passed a resolution calling for 85 percent reductions in CFCs and halons (London Observer 1988). At that point, Thatcher became personally committed to protecting the ozone layer, delivering a speech before the Royal Society that marked a sharp turning point in her government's policies on global environmental problems (Thatcher 1988). In her speech, she announced that she would convene a major international conference in London the following year to promote an 85 percent global reduction of CFCs. 5 Jonathan Porritt, executive director of Friends of the Earth, declared, "Blimey, the speech is impressive, a milestone for the environment" (Washington Post 1989b). Once the United Kingdom had endorsed decisive action, the way was paved for the EC to follow suit.
At roughly the same time, industry's independent review of the Ozone Trends Panel findings was released. Once the conclusions had been substantiated, industry as a whole followed Du Pont. In September 1988, within days of each other, the European Fluorocarbon Technical Committee (EFTC), industry's liaison with the EC and the national governments, ICI, and the U.S.-based Alliance for a Responsible CFC Policy all announced their support for a phaseout on the substances controlled in the Montreal Protocol.
The Ozone Trends Panel report inspired a substantial shift in the terms of public discourse on ozone, suggesting that more stringent international regulatory action would be required. No longer was precautionary action possible; damage was already occurring, and the question was now one of slowing the rate of future destruction. With new consensual knowledge implicating CFCs, the dominant policy discourse on ozone shifted from one rooted in the precautionary principle to one seeking damage control. But that knowledge only came to be universally accepted after the primary skeptics were persuaded by their own trusted informational sources. And the economic value of the new knowledge varied widely within the CFC industry, with Du Pont apparently having the most to gain from it.
Implementing the Discourse of Damage Limitation
In October 1988, UNEP sponsored meetings in The Hague to focus world attention on the ozone problem and the need to strengthen the Montreal Protocol. At that meeting, the following four panels that would provide the knowledge base for revising the treaty were established: scientific, environmental effects, economic, and technical. 6 The panels, involving hundreds of experts from all over the world, represented a pioneering effort in knowledge production aimed at solving a global environmental problem. Panel members had known each other, in some cases, for many years and had developed a strong, practical network for information sharing. The CFC producers were intentionally excluded from the technology panel not only because of a general distrust of them but also to encourage nonchemical alternative technologies (interviews with Stephen Anderson and Robert Watson).
The Hague meeting also called attention to the fact that ratification was proceeding slowly, particularly in the EC. Although several EC states had already decided to surpass the Montreal Protocol's requirements and phase out CFCs by 2000, the formality that all member states, plus the commission, must ratify simultaneously delayed ratification until December 1988, just in time for the treaty to take effect on January 1, 1989.
While the EPA's Lee Thomas called for a CFC phaseout in September 1988, election-year politics virtually guaranteed that official action would be delayed. The Natural Resources Defense Council (NRDC), seeking to hasten things, launched its Atmospheric Protection Initiative and filed a new lawsuit against the EPA (NRDC 1988:4). The NRDC initiative was bolstered by new findings that an ozone hole was forming over the Arctic (New York Times 1988d).
Despite the dramatic ozone losses, the dominant view was that ozone-depleting chemicals could only be banned if substitutes were available. Thus, the perceived availability of CFC substitutes had a strong effect on discourse about ozone. For some sectors, especially aerosols and foam blowing, alternatives to CFCs were relatively simple to find (Christian Science Monitor 1988; Wall Street Journal 1988a). While producers, especially Du Pont, were pressing ahead in the search for new compounds, some user industries were quite disturbed by the situation (Monastersky 1988:234). The refrigeration and air conditioning industries, in particular, were facing new federal energy-efficiency standards just as proposals to ban the highly efficient CFCs were being broached. Potential substitute chemicals and alternative technical designs were likely to conflict with the goal of conserving energy to curb global warming. Hydrofluorocarbons (HFCs), which pose no threat to ozone because they contain no chlorine, were possible chemical substitutes, but they are potent greenhouse gases. In the melodramatic words of one industry representative, the situation had "all the earmarks of a calamity" (Corcoran 1988:113).
Industry emphasized the cost of the transition to new chemicals - some $5.5 billion between 1990 and 2010 in the United States alone, rising to $27 billion by the year 2075 (interview with Kevin Fay). But those amounts paled in comparison to the EPA's projected cost of three million additional skin cancer deaths in the absence of the Montreal Protocol. In monetary terms, to say nothing of less tangible factors, the treaty would save U.S. citizens $6.5 trillion in medical costs by 2075 (interview with Stephen Seidel).
Amid such discussion, along with new reports of severe ozone depletion over the Arctic (Science 1989:1007), preparations were under way for Prime Minister Thatcher's London Conference on Saving the Ozone Layer. In February, Canada announced that it would ban CFCs and halons within ten years (San Francisco Chronicle 1989). On March 3, 1989, the EC's Environment Council unexpectedly voted to eliminate CFCs completely by the end of the century (New York Times 1989a). With the British change of heart, France, maintaining that unilateral reductions would not save the ozone layer and would only benefit non-European companies, had found itself isolated in its opposition to stringent reductions. But France relented, apparently favoring its political interests over its economic interests. President Mitterrand, taking a leadership role in efforts to prevent global climate change, was cosponsoring an international conference on the global environment in The Hague later that month. 7 Had his government blocked efforts in the EC to save the ozone layer, Mitterrand would have been in an embarrassing position at that conference (Jachtenfuchs 1990:271). The day after the EC vote, President Bush announced that the United States would also ban all production of CFCs by the end of the century and would also eliminate halons (Washington Post 1989a). The stage was set for a unified push by the United States and the EC, at loggerheads during the Montreal Protocol negotiations, for a total phaseout of CFCs.
Although the U.K.-sponsored conference was not part of the official treaty revision process, it was cosponsored by UNEP, and it paved the way for the First Meeting of the Parties, to be held two months later in Helsinki. One hundred twelve nations participated in the conference, the first high-level international gathering on ozone since 1987; just eighteen months earlier, only half that number of countries were represented at Montreal. 8 The London Conference laid out the main themes and potential stumbling blocks for the ensuing revision process. In sharp contrast to earlier meetings, a consensus now prevailed on the science of ozone depletion as well as on the need to eventually eliminate CFCs. The treatment of developing countries, which hitherto had been considered a minor issue, became a central concern. While Thatcher was unsuccessful in overcoming objections from the Soviet Union and developing countries to an 85 percent reduction of CFC and halon emissions, she did garner commitments from twenty additional countries to ratify the Montreal Protocol (New York Times 1989c).
The strongest and most ominous objections were raised by India and China, two countries that had not signed the treaty. They argued that developing countries should not have to forgo necessities like refrigeration in order to help solve a problem caused by industrialized countries. Nor should they, they argued, be forced to pay higher prices for substitutes, thereby contributing to the profits of the chemical companies that had caused the problem. China and India submitted an innovative proposal that a fund be established to finance the transfer of substitute technology to developing countries (Christian Science Monitor 1989). That proposal was destined to become a dominant theme during discussions of treaty amendments during the next year.
Two months after the London Conference, the First Meeting of Parties was held in Helsinki. Although the treaty could not legally be revised at that meeting, the delegates were able to agree on a nonbinding declaration and a concrete timetable for the assessment process. The EPA made a key presentation on future chlorine concentrations in the atmosphere according to various emission scenarios for CFCs and other chlorine-containing chemicals. (The scientific underpinnings of that presentation, and its implications for policy discourse, are discussed in greater length below.) On the basis of that presentation, many delegations supported the addition of methyl chloroform and carbon tetrachloride to the list of the treaty's controlled substance (New York Times 1989d). The Helsinki Declaration called for a phaseout of CFCs no later than the year 2000, a phaseout of halons with no specific target dates, and control and reduction of other ozone-depleting chemicals as soon as feasible (UNEP/OzL.Pro.1/5).
The other major issue at the Helsinki meeting was the contentious one of financial and technical assistance for Article 5 countries. Developing countries, backed by the Nordic countries and New Zealand, proposed that a fund be established along the lines of China and India's suggestion at the London conference, but the major donor countries rejected the proposal. In the end, a special working group was established to formulate recommendations on a funding mechanism to be considered by the Second Meeting of Parties the following year (UNEP/OzL.Pro.1/5:20).
Even as the delegates were meeting as Helsinki, the nearly five hundred scientists and technical experts on the four assessment panels were assembling their reports. Almost two thousand pages of work were completed and peer-reviewed during the summer and then consolidated into a fifty-page summary, known as the "Synthesis Report" (UNEP/ OzL.Pro.WG.II[1]/4). That document, presented to the Open-Ended Working Group of the Parties at its Geneva meeting in November 1989, became the basis for the first treaty review. Perhaps out of necessity, since the bulk of the world's technical expertise relevant to the ozone problem resides in the North, the four review panels were heavily dominated by developed countries. Each panel was chaired by either an EC or a North American country, with representatives from the United States serving as either chairs or cochairs on three of the four panels. Nonetheless, despite the apparent potential for dissension, there was surprisingly little debate about the reports.
Of the four assessments, the scientific one was clearly the most critical; estimates of present and future ozone depletion were the driving force behind the treaty revision process. More of the Synthesis Report was devoted to the science assessment than to the other three assessments combined. The observational findings, including data on the Antarctic ozone hole, perturbed Arctic chemistry, and long-term ozone losses in the Northern Hemisphere, were largely drawn from the Ozone Trends Panel report. These findings revealed the defects in the gas-phase computer models used to guide the Montreal Protocol, sparking laboratory studies and model simulations of surface-induced chemical processes. One important study, mentioned in the 1989 Synthesis Report, concluded that a major volcanic eruption could spew large quantities of sulfuric acid particles into the stratosphere, thereby causing on a global scale the same sort of chemical reactions taking place on the ice clouds of Antarctica (UNEP/OzL.Pro.WG.II[1]/4:6; New York Times 1989e:C-4).
With the computer models cast in doubt, the chlorine-loading approach, analyzed in the previous chapter in relation to the Antarctic ozone hole, became the dominant way of framing the scientific information. As discussed earlier, that discursive strategy was based on a rather simplistic calculation of the atmospheric abundance of chlorine given various emission scenarios. The chlorine-loading approach was the basis of the statement that an immediate 85 percent reduction in CFC emissions was required simply to stabilize atmospheric concentrations of chlorine at existing levels. That statement drew attention to the long atmospheric lifetimes of CFCs, debunking the intuitively appealing notion that a simple freeze would be sufficient to stabilize chlorine concentrations at existing levels. The 85 percent figure, first cited by the EPA and environmentalists throughout the Montreal Protocol talks, was later embraced by Prime Minister Thatcher and others who wanted to strengthen the treaty.
Once the Antarctic ozone hole had been definitively linked to CFCs, especially in the absence of models accounting for the phenomenon, the chlorine-loading methodology became the most credible discursive strategy for analyzing alternative policy proposals. The observations of the hole, along with perturbed Arctic chemistry and long-term ozone decreases, led to the recognition of major gaps in the computer models used for past assessment studies (UNEP/OzL.Pro.WG.II[1]/4:5). If chlorine was the culprit, then the logical conclusion was that chlorine concentrations should be reduced to levels lower than those at which the hole appeared. The Antarctic ozone hole emerged when concentrations exceeded two parts per billion (ppb) (preindustrial levels of atmospheric chlorine were only 0.6 ppb [UNEP/OzL.Pro.WG.II(1)/4:28]). 9 Eliminating the hole, therefore, would require decreasing chlorine levels to below 2 ppb.
It should be noted that all participants seem to have assumed that Antarctica's ozone will recuperate if chlorine concentrations return to less than 2 ppb. This assumption, although a reasonable conjecture given past experience, may be erroneous; the nonlinearity of ozone depletion processes indicates that other outcomes are possible. Nonetheless, the entire treaty review process, from 1988 until 1992, was based upon this assumption.
The chlorine-loading approach was not wholly uncontroversial. The British CFC industry continued to harbor objections to a CFC phaseout, framing those objections in terms of skepticism regarding the chlorine-
|
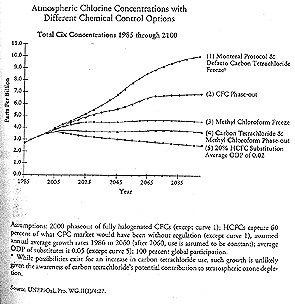
John Hoffman of the EPA, one of the original proponents of this methodology, began working out proposals to revise the treaty based on the chlorine-loading approach the day after he returned from Montreal in 1987 (interview). He calculated that the chlorine abundance under the provisions of the Montreal Protocol would skyrocket to 11 ppb by the end of the next century (see figure 5.1). Even with a complete phaseout of CFCs by the year 2000, chlorine-loading values would still reach an astounding 9 ppb. Hoffman saw that lower levels could only be achieved by regulating carbon tetrachloride (CCL4) and methyl chloroform (CH3CCl3, also known as 1,1,1-Trichloroethane). Even then a complete phaseout, in addition to eliminating CFCs, would only bring the peak chlorine levels down to 4 ppb; the 2 ppb target would not be achieved for over eighty years.
Recognizing that recuperation of the ozone layer would require controlling emissions of chemical substitutes for CFCs, Michael Prather and Robert Watson of NASA expanded upon Hoffman's work (interview with Robert Watson). Their work, eventually published in Nature, served as the scientific basis for the treaty revision process and was
|
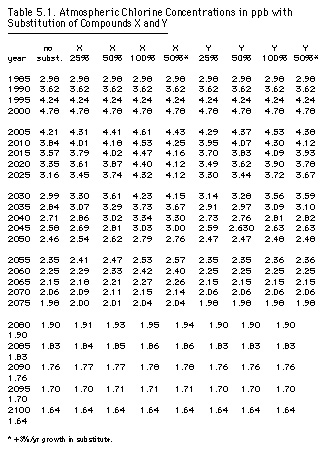
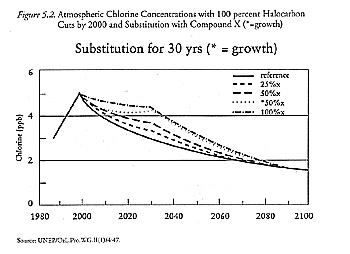
The Synthesis Report considered the transient use of substitutes by examining scenarios with a range of phaseout dates for hypothetical chemicals chosen to mimic actual substitutes. Compound X has a fifteen-year lifetime (like HCFC-22), a single chlorine atom, and a steady-state chlorine-loading factor of 0.10 relative to CFC-11; compound Y has a six-year lifetime (like methyl chloroform), with one chlorine atom and a steady-state chlorine-loading factor of 0.04 (UNEP/OzL.Pro.WG.II[1]/4:32). Table 5.1 presents the chlorine concentrations by date for one scenario: elimination of CFCs, methyl chloroform, and carbon tetrachloride by the year 2000 and various substitute phaseout schedules. Figure 5.2 depicts this hypothetical scenario graphically for compound X.
The Synthesis Report also looked at other approaches, including revised values for ozone depletion potentials (ODP) and global warming potentials (GWP). As discussed in chapter 4, ODPs are calculated with a chemical model of the ozone layer and, in contrast to the relative chlorine-loading values, they include the effectiveness with which the gas releases its chlorine in the stratosphere. But the models that
|
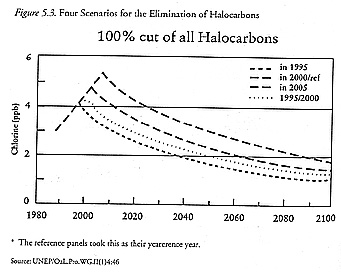
Two parameters, both of which can be depicted either graphically or by analogy, became the focus of concern: lowering the peak atmospheric chlorine concentrations and shortening the length of time before which concentrations would return to 2 ppb. The first problem came to be known as "peak shaving"; as figure 5-3 shows, lowering the peak chlorine concentrations is analogous to slicing pieces off the top of a cake. The
|
Although the chlorine-loading approach was used both before and after the Montreal Protocol, the purposes for which it was used were very different. Prior to 1987, the approach, with its emphasis on the long atmospheric lifetimes of CFCs, was used to inspire a long-term perspective; the problem was framed in terms of stabilizing atmospheric concentrations of chlorine. But by 1989, amid the growing recognition that the world might have to adjust to life without CFCs, the question then became one of peak shaving - i.e., figuring out how to lower the peak chlorine concentration, which would occur sometime around the year 2000, as quickly as possible. The emphasis therefore shifted from the ozone-depleting chemicals with long atmospheric lifetimes to those with short lifetimes. Compounds with the shortest lifetimes, like methyl chloroform, which lasts only six years in the atmosphere, became a major focus because the benefits would be reaped most quickly. Thus, the same approach that was used as the basis for a long-term perspective before Montreal was now used in support of measures with a more short-term advantage. 10 The knowledge brokers responsible for framing the science were quite aware that the same approach could be used for both purposes and intentionally applied that knowledge to the development of policy options (interviews with John Hoffman and Robert Watson).
Carbon tetrachloride, one of the cheapest and most toxic organic solvents, has an ODP significantly greater than any of the five regulated CFCs. With an atmospheric lifetime of fifty years, this compound is an extremely potent ozone destroyer (UNEP/OzL.Pro.WG.II[1]/4: 27). Yet it was overlooked in the Montreal Protocol; in fact, prior to 1988, there was virtually no mention of it in the context of saving the ozone layer. The reasons for its omission were essentially political. Prior to 1988, ozone depletion was tacitly defined as a problem for the industrialized countries; the principal adversaries in the Montreal Protocol negotiations were the USA and the EC. Because of its extreme toxicity, carbon tetrachloride had been banned or severely restricted in most industrialized countries, so its omission from the negotiations seems to have been unintentional. Yet the negotiators overlooked the fact that the low cost of carbon tetrachloride - only twenty-five cents per pound - made it an attractive solvent in developing countries (Makhijani, Bickel, and Makhijani 1990).
Another oversight related to bromine, a far more potent destroyer of ozone on a per molecule basis than chlorine. Halon-1301, for instance, has an astronomical ODP of 10.0. The scientific assessment Panel had stated that closing the seasonal Antarctic ozone hole would also require a phaseout of the halons. It did not, however, make any recommendations regarding methyl bromide, a less significant source of atmospheric bromine; that compound did not become a source of widespread concern until the subsequent 1991 assessment.
Thus, relying on the chlorine-loading methodology, the 1989 science review indicated that recuperation of the ozone layer would require major revisions of the Montreal Protocol, including a ban on CFCs, methyl chloroform, and carbon tetrachloride and only a transitional usage of HCFCs. Even if all anthropogenic sources of chlorine were eliminated immediately, atmospheric concentrations of chlorine would remain above 2 ppb for many decades to come; hence, even in the best of circumstances, the Antarctic ozone hole would recur for the foreseeable future. Only the chlorine-loading method of framing the science generated such conclusions, but the urgency of the situation, along with the obvious limitations of the models, gave credence to this approach over the alternatives.
With the growing sense of urgency, however, it was easy to forget that the health and environmental effects of increased ultraviolet radiation (UV-B) were the real threat - not ozone depletion per se. Yet, while funding for stratospheric science had increased sharply, funding for research on the effects had essentially remained at pre-Montreal levels (interviews with Stephen Seidel and Robert Watson). As a result, the 1989 environmental effects assessment contained little new information about the impact of increased UV-B on terrestrial plants, aquatic ecosystems, tropospheric air quality, and materials damage beyond that found in the EPA's 1987 study (EPA 1987a). In terms of human health, the emphasis continued to be on skin cancer and cataracts, despite the fact that increased UV-B radiation could have serious immunological consequences (interview with Margaret Kripke). The emphasis on skin cancer, which primarily afflicts fair-skinned people, seems particularly surprising at a time when developing countries were becoming key players in the policy debates. Yet there were few attempts to shift the emphasis, and there were virtually no challenges to the science.
This apparent anomaly can only be explained with reference to the prevailing discursive practices on ozone. Rational models of decision making would predict that, since increased UV-B radiation was the real problem, there would have been a substantial research effort into the health and environmental effects of ozone depletion. And past experience would suggest at least the possibility that developing countries would frame their objections to the treaty and the proposed revisions in scientific terms, perhaps arguing that skin cancer was not their concern. Yet neither of these possibilities occurred; in fact, there was almost no attention paid to this section of the Synthesis Report.
Instead, the scientific assessment was the driving force behind the revision process. The dominant perception was that something unprecedented and potentially catastrophic was happening in the stratosphere and that it was not necessary to quantify precisely the likely effects of that event. The graphs and charts made it obvious to the participants that drastic action was required; the discourse of damage limitation became predominant in policy discussions almost automatically. 11 In the words of one participant, the prevailing attitude among members of the Open-Ended Working Group and other participants was "that of the man on the street: 'Don't fool with Mother Nature' " (interview with John Hoffman). This sentiment also informed the precautionary discourse embodied in the Montreal Protocol, but with the mounting evidence of impending catastrophe, it was no longer controversial. By the time of the 1989 Synthesis Report, the discourse of damage limitation had become universally accepted. Only the precise control measures had yet to be determined by the Second Meeting of the Parties in June 1990.
The 1989 technology assessment provided information on the technical feasibility of reducing ozone-depleting substances. Most important was the finding that, by the year 2000, the five controlled CFCs and carbon tetrachloride could be phased down by at least 95 percent and methyl chloroform by at least 90 percent (though this conclusion was most controversial). The panel disagreed on whether a complete phaseout of halons was technically achievable by 2005. There was a consensus that 30 percent of the existing CFC market would be captured by HCFCs and 10 percent by HFCs, with the remaining 60 percent of demand satisfied by product and process substitutes (UNEP/OzL.Pro.WG.II[1]/4:10–11).
Although the issue of technology transfer and assistance to developing countries would later dominate the treaty revision process, the 1989 economic assessment was not particularly controversial. The panel acknowledged the need for special provisions for developing countries to obtain the more expensive chemical substitutes but made no specific recommendations. The panel also calculated that the long-term economic benefits of safeguarding the ozone layer, while difficult to quantify, would far outweigh the short-term costs of reducing CFC and halon usage. However, as industry was prone to emphasize, a very rapid phaseout (much faster than ten years) would be far more costly because of capital abandonment (UNEP/OzL.Pro.WG.II[1]/4:12–13). Perhaps because of poor communication between the economics panel, largely drawn from academic circles, and the science and technology panels, the economic review did not explore the crucial issues of reducing methyl chloroform, carbon tetrachloride, and HCFCs.
The chemical industry reacted negatively to two implications of the Synthesis Report: that HCFCs should be used only as transitional substances and that methyl chloroform should be sharply reduced. At UNEP's Nairobi meeting in August 1989, where the Synthesis Report was first discussed, Du Pont distributed literature promoting industry's position that "potential regulation of HCFC and HFC alternatives will delay or prohibit investments and the transition away from CFCs" (Du Pont 1989:6). However, by the June 1990 Meeting of the Parties, U.S. industry backed away from this position, causing a split with its European counterparts. Recognizing that chlorine concentrations would only return to pre–ozone hole levels if HCFCs were regulated, American industry saw that definite phaseout dates were preferable to a vague threat of eventual regulation. Thus, at the London meeting, ICI continued to distribute brochures opposed to including HCFCs on the list of controlled substances, while the Alliance for a Responsible CFC Policy circulated literature advocating "prudent HCFC phaseout dates" (ICI 1990; Alliance for a Responsible CFC Policy 1990:3). U.S. industry, however, remained adamant that any phaseout schedule adopted in London should be compatible with the thirty- to forty-year lifetime of equipment using HCFCs (interview with Kevin Fay).
Industry on both sides of the Atlantic was also disconcerted by the implication that methyl chloroform, a metals and electronics solvent and a potential substitute for CFC-113, might be added to the list of controlled substances. Industry felt that the technology panel's work on methyl chloroform was weak and done too hastily, a sentiment shared by others who were not necessarily sympathetic to industry's aims (interviews with Stephen Anderson and Stephen Seidel). Yet at least some environmentalists had earlier recognized that methyl chloroform was a significant part of the ozone problem (Makhijani, Bickel, and Makhijani 1990). One obstacle to consensus was that the issue brought in a new set of players not conversant with the ozone problem. ICI, which had been active in the international negotiating process from the beginning, was a primary producer of methyl chloroform in Europe. But Dow Chemical, with no previous involvement in the issue, was the primary producer both in the United States and the world, accounting for nearly half of global production. Unlike Du Pont, which had two atmospheric scientists working only on the ozone problem, Dow Chemical had none. Had Du Pont, rather than Dow, been the top producer, perhaps events would have transpired differently (interview with Stephen Seidel). In any case, ICI and Dow Chemical joined together to lobby against strong controls on methyl chloroform.
Once again, through creative framing and the inclusion of partial information, scientific knowledge was used to promote parochial interests. Industry, with Japan's support, 12 emphasized methyl chloroform's low ozone depletion potential - only about 0.1 - and its short atmospheric lifetime of approximately six years. Reminiscent of the CFC industry's statements in 1986, the methyl chloroform industry also claimed that production was declining, a claim that was later refuted (interview with Stephen Seidel). Industry itself was surprised when the 1989 production figures were announced in April 1990, just two months before the Meeting of the Parties, showing a major increase over 1988 levels (interview with Mike Harris).
At the Geneva working group meeting in November 1989, where the final draft of the Synthesis Report was considered, three industrial associations argued that the maximum technically feasible reduction of methyl chloroform was 23 percent by the year 2000. This figure was at odds with the technology assessment, which concluded that a full phaseout was achievable by the same date. The science panel responded to industry's claim, noting that even a 20 percent reduction in 1986 emissions by the year 2000 would still increase chlorine concentrations by 0.4 ppb and would delay recovery of the ozone hole by thirty years. Because of the huge quantities being produced - as much as all the CFCs combined - methyl chloroform was responsible for approximately 16 percent of the total anthropogenic chlorine loading (UNEP/OzL.Pro.WG.II[1]/7:40). In the end, the science assessment overshadowed industry's objections; in particular, the graphs included in the report provided strong evidence in favor of a phaseout (see the figures in this chapter, especially figure 5.1).
By 1990, with the Second Meeting of the Parties approaching, industry saw the inevitable and relented. The Nordics had proposed a phaseout date as early as 1995, and the U.S. Clean Air Act called for regulation of methyl chloroform domestically. Within less than a year, Dow Chemical and ICI shifted from vociferous opposition to reluctant support of a phaseout. Many observers were critical of Dow for its initial recalcitrance, particularly in contrast to Du Pont's apparently cooperative attitude. Yet Dow Chemical arrived at its position of support for the regulators in only one year, in contrast to Du Pont's ten-year journey to a comparable position (interview with Stephen Anderson).
Proposals to reduce sharply or eliminate carbon tetrachloride were not nearly so factious (UNEP/OzL.Pro.WG.II[1]/4:27). As noted earlier, most Western industrialized countries had already severely restricted its use, except as a feedstock for CFCs. But the industrialized countries were shocked to learn how widely used the compound was in the Soviet Union, Eastern Europe, and developing countries (interview with Eileen Claussen). The Soviets led the effort to salvage carbon tetrachloride, but they were unable to galvanize sufficient support among other countries. 13
Although a broad consensus existed on the need to phase out CFCs, to reduce halons more quickly, and to add carbon tetrachloride and methyl chloroform to the list of controlled substances, the specific dates and interim reduction steps proved to be more contentious. At the Nairobi meeting where the Synthesis Report was reviewed, only the Nordic group called for concrete steps to bring chlorine levels below those at which the Antarctic ozone hole formed: a CFC and methyl chloroform phaseout by the year 2000, with 50 percent cuts before the mid-1990s; a carbon tetrachloride phaseout by the mid-1990s; and controls on all substances with ozone depletion potentials greater than 1 percent of those of the CFCs. Environmentalists lobbied their governments to adopt the Nordic position (FOE 1989b:3). Most countries supported a CFC phaseout by the year 2000 but would not commit to eliminating the other chemicals. The Open-Ended Working Group was compelled to meet on a bimonthly basis, to delegate many duties to smaller groups, and to establish a Bureau of the Parties to formulate specific recommendations for control provisions (UNEP/OzL.Pro.Bur.1/2).
As it became clear that CFCs were likely to be eliminated, developing countries became increasingly vocal, although only a handful of them were parties to the treaty. Article 5 granted them a grace period of ten years to reduce their CFC usage by 50 percent, but CFCs were likely to become scarce and expensive soon in light of the proposed treaty revisions. Developing countries did not want to increase their dependency on a family of obsolete chemicals, nor did they want to pay far higher prices to the industrialized countries' chemical companies for substitutes. Yet the science assessment had demonstrated that, even with full compliance under the Montreal Protocol, chlorine concentrations would rise to an astronomical 11 ppb, an unacceptable scenario for developing and industrialized countries alike.
As I mentioned earlier, the developing countries did not state their reservations in scientific terms, even though they were barely represented on the science and environmental panels and so could have challenged the veracity of these panels' findings on that basis alone. Other arguments were also there to be made: ozone depletion was occurring at the poles and the upper latitudes, not over the tropics, and skin cancer was not likely to become a health problem for non-Caucasian populations. The recurring Antarctic ozone hole, with its dilution effect throughout the Southern Hemisphere, certainly drew the attention of the South. But it should be remembered that only 7.5 percent of the world's population lives in the Southern Hemisphere (United Nations 1989). A few countries - namely, Argentina, Chile, Australia and New Zealand - are directly at risk because of the Antarctic hole, but the bulk of developing countries' population is concentrated in the tropical regions of the Northern Hemisphere, the area of the globe least at risk.
But developing countries neither disputed the findings of the science panel nor questioned whether their own populations would be vulnerable to the health and environmental consequences of increased ultraviolet radiation (interviews with Mohammed Ilyas and M. Margarita Prendez). Perhaps this can be explained by their relative lack of scientific infrastructure; clearly, stratospheric research is not a priority for poor countries. Or perhaps the developing countries were simply persuaded by the information contained in the Synthesis Report. But that alone does not seem sufficient to explain their almost automatic acceptance of the science. Again, it seems that the discourse of damage limitation had become so ubiquitous and had gained so much momentum that there was virtually no effort to supplant it with a alternative discourse, even from those - industry and the developing countries - with the greatest interest in promoting something different. Instead, a new wave of environmental concern was spreading, even among many of the developing countries (see FOE 1989a).
With a consensus that CFCs would be eliminated, developing countries focused their energies on the problems of financial compensation and technology transfer. During a lengthy EPA visit in 1988 to China, the sole focus of discussion was CFC substitutes and the economics of technology transfer (interview with Eileen Claussen). At the Nairobi meeting of the Open-Ended Working Group in August 1989, developing countries stated that they could only abide by the terms of the Montreal Protocol if they received technical and financial assistance. They reiterated the desirability of establishing a multilateral trust fund administered by UNEP and funded by industrialized countries over and beyond existing aid programs (the principle of "additionality"), and they sought equal access to new chemical substitutes without incurring economic penalties (the principle of "preferential and non-commercial technology transfer") (UNEP/OzL.Pro.WG.I[2]/4).
These principles were quite controversial. At least some participants from industrialized countries feared that developing countries would use environmental problems as an excuse to demand a global redistribution of wealth, and some even suspected that this was part of Mostafa Tolba's agenda (interview with Ed Shykind). Some industrialized countries, most notably the United States, the U.K., and Japan preferred to provide assistance through an existing institution like the World Bank, a proposal viewed with suspicion by developing countries, which believed they could exert more influence with UNEP. One major uncertainty was how large the fund should be, a calculation that required accurate information on present and future CFC demand, as well as on the future cost and availability of chemical substitutes. A controversial study done by a Netherlands consulting firm was cited by Mostafa Tolba at the August meeting in Nairobi. That study estimated the cost to developing countries to be $400 million annually for ten years (Tolba 1989b). Because of the tremendous uncertainties entailed in making reliable estimates, the delegates at the Nairobi meeting decided to commission feasibility studies in sample developing countries to identify their needs and the eventual cost of complying with the treaty. Developing countries, however, were disappointed, viewing the move to study the issue as an attempt by industrial nations to avoid committing actual funds (FOE 1989b:2).
The question of technology transfer and financial compensation became the focus of debate between North and South until the 1990 London Conference of the Parties. Between late 1989 and June 1990, one working group or another was meeting almost constantly, with this issue frequently dominating the agenda. India, for instance, expressed a desire that all CFC production should be stopped, but only after developing countries received alternative technology (International Environment Reporter [IER] 1990). 14 In the meantime, Tolba asked the EPA and other environmental agencies in industrialized countries to coordinate "country studies" to ascertain the cost of the transition to CFC substitutes. The sample countries included Mexico, Brazil, India, China, India, Venezuela, Kenya, and Malaysia.
At the February 1990 meeting of the Open-Ended Working Group, the results of two general studies on the costs of developing countries' compliance with the Montreal Protocol were discussed. One study was done by a British firm (Markandya 1990), and the other was done by an Indian firm (Pargal and Kumar 1990). The two research teams reviewed each other's data and methodologies and presented a joint report on their areas of agreement and disagreement. They agreed on some broad principles regarding what should be considered legitimate costs, but they disagreed on the key factor of demand growth projections. As in the Montreal Protocol negotiations, growth projections reflected the political and ecnomic interests of the parties making the projections. Predictably, the Indian report foresaw higher future demand for CFCs and their substitutes in developing countries and therefore concluded that a larger fund would be required. But because the executive summary of the Indian report was vague, containing no specific cost estimates, the British report was more authoritative. That study's executive summary proposed an initial allocation of $200 to $300 million for the first three years. The credibility of the British report was bolstered when the EPA estimated that the incremental costs to Article 5 parties would be approximately $100 million for the next three years, and $100 to $200 million more if China, India and other nonsignatory parties were to sign (UNEP/OzL.Pro.WG.II[2]/7). With this remarkable concordance between studies, the amount of $200 to $300 million over three years came to be bandied about as a plausible amount for the fund (UNEP/OzL.Pro.WG.IV/8). 15
That figure could be little more than conjecture, however. As late as the March meeting of the Open-Ended Working Group, there was no reliable data on 1986 levels of CFC consumption in developing countries. Several delegations proposed that UNEP should hire a consultant to present a "best available estimate," but others doubted whether such information could be prepared in time for the May funding meeting (UNEP/ OzL.Pro.WG.III[1]/3:7). If past levels of consumption were unknown, then clearly predictions of future levels were necessarily that much more obscure. Yet, because of the political deadline imposed by the upcoming Meeting of the Parties in June, the participants were compelled to accept the figures presented in the EPA and Markandya studies.
The discussion of preferential and noncommercial access to substitute technology raised the thorny issue of intellectual property rights. Industry was loath to invest in substitute technology only to have it donated to developing countries; yet without new technology, the goals of the Montreal Protocol, particularly an amended protocol, could not be met. The International Chamber of Commerce (ICC) felt that this bid to mandate technology transfer by treaty could set a dangerous precedent; governments, it claimed, have no authority to compel companies to share their research and technology on a noncommercial basis (ICC 1990). Moreover, as some industry representatives pointed out, chemical substitutes need not be the focus of concern; technologies and processes to reduce, conserve, and recycle were already available to reduce developing countries' demand for CFCs. Building a domestic manufacturing base for chemical substitutes, they argued, should be a low priority, especially when nobody was certain what the new compounds would be (interview with Tony Vogelsburg; ICC 1990).
The principle of additionality was not particularly controversial until the eleventh hour. Just before the special funding meeting in May, the United States announced that it would only support a special funding mechanism within the World Bank if no additional donor contributions were required. The rationale for this position was that the requirement of additional donor contributions would set a dangerous precedent, particularly as the much larger problem of greenhouse warming was gaining attention. The U.S. decision was made by John Sununu, White House Chief of Staff, and Richard Darman, budget director, over the objections of EPA administrator William Reilly (Washington Post 1990a). This was the first time that the United States had raised serious objections to the principle of additionality (Tolba 1989b).
Domestically, the reaction was quite negative (Time 1990; Washington Post 1990b). The reversal was characterized as cynical and irrational; the U.S. share of the CFC fund would be a paltry $8 to $25 million per year, compared to a savings of billions in medical costs. The U.S. chemical industry, which stood to gain from the fund, pointed out that the U.S. contribution would be a tiny portion of the estimated $5.7 billion that the government stood to collect in taxes on windfall profits (New York Times 1990b; see also DeCanio and Lee 1991). Critics also pointed out that the new policy would undermine American environmental diplomacy, casting doubt on the USA's reliability as a negotiating partner. Moreover, the decision, emanating from the Office of Management and Budget and the White House chief of staff, sent the message that the nation's top environmental experts and diplomats were to be excluded from the process (Pell 1990). Legislation was introduced in both houses of Congress to fund the U.S. contribution in case the Bush administration maintained its position (IER 1991f:637).
Internationally, the response was equally critical, in both industrialized and developing countries. At the May funding meeting in Geneva, the other key donor countries expressed their support for the fund, and a representative of the World Bank stated that institution would participate only if the principle of additionality were adopted. China and India declared that they would not sign the treaty without the guarantee of additional funds (UNEP/OzL.Pro.WG.III[2]/3). Members of the U.S. delegation were put in the awkward position of having to retract their support for policy recommendations that, in some instances, they themselves had authored (interview with Eileen Claussen).
This incident bears a striking resemblance to the "hats and sunglasses" episode within the Reagan administration during the Montreal Protocol negotiations. In both cases, ideologically oriented cabinet members who were not directly involved in the negotiations sought to foil the efforts of the EPA and the State Department. And both episodes resulted in significant political embarrassment for the respective administrations. Unlike the first episode, which resulted from a simplistic interpretation of scientific information, the funding proposal, while framed in terms of fairness, was based more explicitly on perceived long-term economic self-interest. It was also more clearly an instance of symbolic politics; if aid additionality were truly the issue, then the United States could simply have chosen to cut its development assistance programs by a few million dollars. Although progress was made at the May meeting of the Working Group, the U.S. position cast a pall over the deliberations.
Updating the Protocol: London 1990
The Open-Ended Working Group met during the week before the Meeting of Parties to finalize a text for the high-level meeting. With over eighty governments and thirty nongovernmental organizations dealing with a myriad of extremely complex issues, the task was unwieldy, and many issues remained unresolved at the end of the week. Two parallel working groups were created to simplify the negotiations: one for control measures and the other for assistance for developing countries. 16 The Working Group was not able to complete its work in time for the ministerial meeting, and some of the decisions reached there did not hold when the ministers arrived. When they did convene, the ninety-five ministers mostly worked in small informal groups, as they had in Montreal, with substantial help from Mostafa Tolba (Benedick 1991:169).
Going into the Second Meeting of the Parties, two sets of issues dominated the debate on control measures: the grim evidence of rapid ozone depletion and industry's progress in finding substitutes and alternatives. These two factors gave a strong impetus to the treaty revision process in London. Regarding the second, progress had been much more rapid than had been expected. Some chemical substitutes were available in all sectors, including refrigeration and air conditioning, the most difficult of all; for some sectors, including aerosols, foam packaging, and electronics solvents, CFCs were already being phased out. Singapore had already reduced its CFC consumption by 60 percent.
Of course, the new chemicals carried greater health and environmental risks than their chemically inert predecessors, as well as a greater financial burden (New York Times 1990c; Manzer 1990). But other avenues, such as recycling and conservation, were both cheaper and safer. Halon and methyl chloroform consumption, for instance, could be cut in half simply through conservation efforts (interview with Stephen Anderson). Overall, the perceived availability of substitutes and alternatives made it easier for delegates to consider stronger control provisions. Of course, the process was circular: the likelihood of stronger controls, prompted by the alarming scientific evidence, also drove the search for alternatives.
At the opening of the London meeting in June 1990, Ivar Isaksen distributed new scientific information to the delegates, including data on unprecedented ozone losses over New Zealand and a surprising decrease of 3 percent in equatorial regions. The Australian delegate reported a 15 percent ozone loss over MacQuarie Island, a region outside the South polar vortex extending over a range that includes one-eighth of the planet. Prime Minister Margaret Thatcher cited a statement by Joe Farman, head of the research team that had discovered the Antarctic ozone hole, that the unexpected events over Antarctica could well be replicated over the Arctic (UNEP/OzL.Pro.2/3). Not surprisingly, this prompted the countries nearest the poles (the Nordic countries, Australia, and New Zealand) to put forth the strongest proposals for controlling ozone-depleting chemicals. The United States had no official position on a CFC phaseout going into the London meeting (interview with Eileen Claussen). 17
Even with the new evidence, most of the proposals considered at the London meetings went no further than those formulated in Helsinki one year earlier. Mostafa Tolba's proposal, modeled on the Helsinki Declaration, served as the basis for the London talks (see table 5.3 for its main elements). Yet, in the interim since Helsinki, many countries -
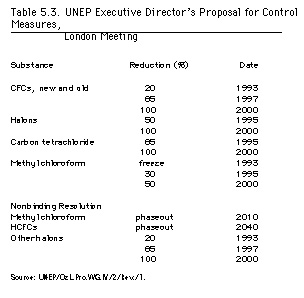
|
Environmental groups, of course, argued that if Germany, one of the major CFC producers, could ban CFCs by 1995, then other countries could certainly do better than 2000. Environmentalists constituted a well-organized presence at the London meeting and proved themselves to be adept at employing scientific knowledge to promote their goals (see FOE 1990a; NRDC 1990). They found the chlorine-loading approach to be particularly useful. Criticizing Tolba's proposal, they argued that it did not even go as far the recommendations implicit in the 1989 Synthesis Report. Tolba's position, if implemented, would allow peak chlorine levels to reach 4.5 ppb, a 50 percent increase over 1990 levels. Worse, even with full compliance not only on the control measures but also on the nonbinding resolution, the levels would remain above 4 ppb until 2050, and the Antarctic ozone hole would not be healed until at least 2080. Environmentalists also joined with Sweden in advocating an early phaseout of methyl chloroform; based upon the scientific evidence, they argued, this was the fastest way of reducing chlorine concentrations in the atmosphere (FOE 1990b:4). They bolstered their position with information from the technology assessment, in which 90 to 95 percent reductions of methyl chloroform were deemed to be technically feasible (Benedick 1991:165).
Industry was also well represented at the London meeting, particularly because the impending treaty revisions involved a broader range of users and producers than did the original protocol. While industry favored a later phaseout date for CFCs - 2000 rather than 1997 - it was not particularly vocal on this issue (interview with Kevin Fay). The major CFC producers were mostly concerned that HCFCs, now considered "transitional substances," not be strictly regulated (interview with Tony Vogelsburg). Du Pont and ICI circulated literature stating that early phaseout dates would discourage commercial investment in substitutes, thereby deferring the far more critical CFC phaseout date. The German CFC industry, in contrast, was silent on the issue of HCFCs, having chosen not to invest in chemicals that would only be transitional and to rely instead on ozone-safe HFCs. On methyl chloroform, U.S. and European industry were both resigned to some reductions, but they diverged in their preferences on the amounts. Under the amended Clean Air Act mandating a methyl chloroform ban, U.S. industry was subject to very different conditions and therefore favored stricter international regulations in order to create a level playing field (FOE 1990b).
On the question of adjustments in the CFC phaseout schedule, the principal adversaries were the EC, with the support of the "strong revision coalition" (the Nordic countries, Canada, Australia, and New Zealand), favoring 1997 and, on the other side, the United States, Japan, and the Soviet Union favoring the year 2000. Developing countries were less concerned with the actual phaseout date than with preserving their ten-year grace period to comply with the protocol's provisions. Ultimately, the least-common-denominator effect, so familiar in international environmental negotiations, prevailed, and the 2000 phaseout date was adopted.
The United States was able to marshal critical information on its behalf: UNEP's own technology assessment had found that a phaseout was technically feasible by the year 2000. This employment of consensual knowledge swayed the balance in the United States' favor (interview with Stephen Seidel). The reduction schedule was changed to 20 percent of 1986 levels by 1993, 50 percent by 1995, 85 percent by 1997, and 100 percent by 2000. Developing countries maintained their ten-year grace period and will not have to reduce CFC use until 2005, when a 50 percent cut will be mandatory. The revisions also closed a potential loophole by including ten "other CFCs" not included in the original protocol because they were not being produced. Thirteen countries broke publicly with the London meeting, issuing a formal declaration pledging to end CFC production by 1997. 19 Britain, when challenged, claimed not to have been asked to sign, but informally declared its support for the 1997 phaseout date.
Environmentalists were quite disappointed with the London CFC revisions (FOE 1990b). They were not, however, so dissatisfied with the outcome on methyl chloroform. Initially, the United States, along with the EC and Japan, backed Tolba's proposal for a 50 percent cut by 2000. 20 The Soviet Union, which did not use the chemical, was joined by West Germany and "the strong revision coalition" mentioned above in pressing for 85 percent reduction by the year 2000. Again, consensual knowledge was brought to bear. The science assessment had shown that reducing methyl chloroform, with its short atmospheric lifetime, would yield the quickest results in lowering chlorine concentrations. And the technology panel had found that 90 to 95 percent reductions were feasible by the year 2000.
But political factors also turned the tide. With a domestic methyl chloroform ban legislated by the Clean Air Act, the U.S. position was not clearly in its own best interest. The Norwegian environment minister, objecting to the nonprecedential language required by the United States on the financial mechanism, consented to include it only if the USA agreed to a methyl chloroform ban. Eileen Claussen enjoyed calling John Sununu, who was far more concerned with the financial mechanism than with the control measures, and saying, "We can get this language, but we have to give up methyl chloroform for it." Sununu, who was only concerned about the financial mechanism, approved the trade immediately (interview with Eileen Claussen). The final agreement was a freeze in 1993, a cut of 30 percent in 1995, 70 percent in 2000, and 100 percent in 2005 (article 2E of the London Revisions, UNEP/OzL.Pro.2/L.6). Thus, an unusual combination of consensual knowledge, domestic U.S. politics, and adroit bargaining on the part of a small state led to action on methyl chloroform that went beyond even the strongest proposal on the table.
While environmentalists were pleased with the results on methyl chloroform, they were extremely displeased with the outcome on HCFCs. A number of governments had already taken measures to curb their usage. In the amended Clean Air Act, the U.S. Senate had set a phaseout date of 2030, and the House of Representative had set a date of 2035. In his proposal, Tolba had suggested 2040. While the CFC producers had reluctantly accepted Tolba's recommendation, their lobbying raised concerns among participants that if HCFCs were formally controlled, companies would not invest in them. Just before the congressional vote on the Clean Air Act, Du Pont announced to the press that it was delaying plans to build HCFC plants pending the outcome of proposed regulations. In the end, however, the EC blocked consensus, and no restrictions on HCFCs were adopted. Instead, a nonbinding resolution was accepted urging efforts to limit the use of HCFCs and setting a voluntary phaseout date of 2040 (annex VII of the London Revisions, UNEP/OzL.Pro.2/L.6). Environmentalists pointed out that the science assessment had demonstrated that controls on transitional substances would be necessary to close the Antarctic hole within the next century. They pledged to bring HCFCs formally into the protocol at the next review meeting, in 1992 (FOE 1990b:8).
There was no organized opposition to Tolba's proposal to eliminate carbon tetrachloride by the year 2000, which was based firmly on the technology assessment's conclusions. The parties could not agree on interim reductions beyond the 85 percent cut in 1995. Some minor differences arose on the baseline date for the new substances, carbon tetrachloride and methyl chloroform; in the end, 1989 was chosen over 1986, the base year for the Montreal Protocol, because the more recent production data was more reliable (interview with Eileen Claussen). Annex II, the protocol amendment covering all new substances, took effect in January 1992.
Given the prominence of the chlorine-loading approach in the debates, it is not surprising that this was the touchstone against which the agreement was measured. The same evidence, however, was used to support different judgments of the control measures adopted at London. Figure
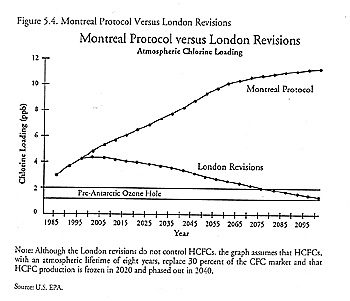
|
For the most part, developing countries were not deeply involved in the debates on control measures. They were, however, quite concerned about the procedure for voting on adjustments. The original procedure adopted in Montreal, requiring that amendments be approved by two-thirds of the parties representing at least 50 percent of consumption, made sense when the primary participants were industrialized countries. However, by the time of the London meeting, it was widely perceived as unfair to developing countries. The new voting procedure required that adjustments be approved by two-thirds of all parties, and simple majorities of developing and industrialized countries (article 2H of the London Revisions, UNEP/OzL.Pro.2/L.6). Article 2, paragraph 4, was changed to grant the additional production allowance only to developing countries to meet their basic domestic needs. Article 4 on trade restrictions was revised in two important ways. Paragraph 2 banning the export of controlled substances to nonparties would now apply to all parties, not just developing countries. And the industrial rationalization clause was adjusted to facilitate the EC's needs (article 4).
In spite of the complexity of the control measures adopted at London, it was the funding mechanism that dominated the meeting. Just prior to the meeting, the United States withdrew its objections to the principle of additionality. But it continued to insist upon several conditions: the addition of language about the fund's nonprecedential nature, a preliminary statement that the ozone problem had been scientifically verified, a permanent seat on the fund's managing committee, and a provision that the majority of seats be held by donor countries (Los Angeles Times 1990b). The second condition implicitly referred to the fact that global climate change had not yet been measured, nor was it likely to be conclusively documented for many years (Intergovernmental Panel on Climate Change 1990). Developing countries, however, opposed the conditions (Los Angeles Times 1990c).
Despite the objections, the first three conditions were met, although the final language, "without prejudice to any future arrangements," was a slightly weaker version of the original U.S. condition (article 10, paragraph 10). As mentioned above, Norway withheld approval of this clause until the United States agreed to a methyl chloroform ban. The USA was granted a permanent seat by the rather awkward method of constituting it as a separate region unto itself out of the seven established among industrialized countries. Developing countries, however, were also divided into seven regions and were thus given voting power on the Executive Committee equal to that of the donor countries.
India and China, neither of whom had yet signed the Montreal Protocol, indicated that they would only sign if they were satisfied with the details of the funding mechanism. India insisted that developing countries should receive not only funding but also the knowledge and patents necessary to produce the new technologies on their own. Du Pont responded with a position paper emphasizing the importance of patent protection in ensuring industry's willingness to invest in a new generation of chemicals (FOE 1990a:11). Malaysia also complained that developing countries were not able to obtain adequate supplies of the increasingly scarce and overpriced CFCs. The developing countries believed that they should not be obligated to abide by the treaty if they were not satisfied with the workings of the financial mechanism. In the final revision, they were not freed from their treaty obligations, but a statement was inserted indicating that their ability to abide by the treaty would depend on the effective workings of the financial mechanism and technology transfer (article 5, paragraph 8).
Because a permanent funding mechanism could not, according to the treaty's terms, operate until 1992, an "Interim Multilateral Trust Fund" (hereafter called the Multilateral Fund) was set up to operate for three years beginning January 1, 1991. The amount of the fund, determined by the results of the country studies, was set at $160 million for the first three years, plus another $80 million should China and India become parties. The fund covers a range of expenses, including projects to retrofit existing CFC-using equipment, to convert CFC plants to substitute production, to import higher-priced substitutes, and to establish recycling programs (UNEP/OzL.Pro.2/L.6). Contributions are to be made according to the U.S. assessment scale. The principle of additionality was putatively adopted, although it is not enforceable. Under certain circumstances, bilateral assistance can count as a contribution to the Multilateral Fund if it provides additional resources (article 10, paragraph 6). Three agencies - UNEP, the World Bank, and the United Nations Development Programme - were designated the implementing agencies of the fund, with the World Bank given the key functions of administration and management (annex IV, appendix 4). 21
China, followed by India, announced that it was satisfied with the results and would sign the treaty. With the important exception of the United States, most delegates applauded the fund. "The U.S. is the only government that doesn't want to admit that the ozone fund is a ground-breaking precedent," said one observer. "It will help gain developing country cooperation on other pressing environmental problems" (FOE 1990d:7).
Ironically, the immediate beneficiaries of the London Revisions would be the world's largest chemical companies (Wall Street Journal 1990). The phasing-out of CFCs meant a guaranteed market for substitutes, a market that favored the chemical giants with their large research budgets and laboratories. But the London Revisions, even if they were considered too weak by environmentalists, went far beyond the Montreal Protocol in limiting global ozone depletion. They continued the movement, launched with the Vienna Convention, toward precautionary action and concretized the discourse of damage limitation based on the chlorine-loading methodology. Moreover, because of the new mechanisms for funding and technology transfer, the treaty was now truly global. Yet, sensing that the revisions might not be sufficient, the participants at the London meeting decided to move up the next scientific and technical review in order to pave the way for additional revisions in 1992.
Further Revisions: Copenhagen 1992
The international effort to save the ozone layer was, more than anything else, driven by information. As one of UNEP's assessment panels observed, "It is information on the extent of ozone damage and the contingent damage costs that motivates the ODS substitution process, and it is information on alternative technologies and processes that makes substitution possible. Furthermore, the effectiveness of the Interim Multilateral Ozone Fund will be built on efficient information flows" (UNEP 1991c:15).
The two primary factors that drove the treaty revisions, the scientific observations of unprecedented ozone losses and the rapid progress in generating alternative technologies, continued to dominate policy discussions in the aftermath of the London meeting. Scientific discourse, framed in terms of the link between atmospheric chlorine concentrations and ozone losses, pointed to the necessity of further controls on ozone-depleting substances. And technological developments highlighted the possibility of implementing such controls. Together, science and technology suggested policies that would concretize the discourse of damage limitation. Reminiscent of events in the immediate aftermath of the Montreal Protocol, by the time of the Third Meeting of the Parties, held in Nairobi in June 1991, new developments suggested that the London Revisions had not gone far enough.
Since the London meeting, only one year before, the situation had become much more critical. The Antarctic ozone hole, which had hitherto evinced a biennial oscillation with weaker holes in even-numbered years, was as bad in 1990 as in 1987 and 1989 (Science 1990). New observations made since the 1989 scientific assessment indicated major ozone losses near the Arctic - as much as 43 percent over Scandinavia (IER 1991a). Just two months before the Nairobi meeting, NASA announced that total column ozone over North America was being depleted twice as fast as had been previously thought. Scientists were uncertain about whether it was sulfate aerosols or polar stratospheric clouds that provided the mechanism for CFCs to deplete ozone in the Northern Hemisphere, but they were certain about the observations (IER 1991b). Sherwood Rowland, coauthor of the original CFC–ozone depletion theory, declared that NASA's data, which used 1978 as a base year, had underestimated the ozone losses. In response, Albert Gore led thirty other senators in urging President Bush to follow the lead of the EC, which had approved in March 1991 a phaseout of CFCs by 1997 (IER 1991c). 22 Environmentalists, calling the new data "frightening," urged their governments to adopt Germany's 1995 CFC and halon ban (IER 1991b).
New technologies, including both new chemicals and new processes, continued to be developed for the approximately thirty-five hundred specific applications using CFCs (interview with Tony Vogelsburg). Every year since the signing of the Montreal Protocol, thousands of users had attended the Substitutes and Alternatives Conference jointly sponsored by UNEP and the EPA. And every year, several important new technologies were announced at the conference (interview with Stephen Anderson). With a guaranteed market for CFC substitutes, industrial firms such as Atochem, ICI, and Du Pont were actively commercializing HCFC-141b, -142b, -134a, and -123 and HFC-134a. Chemical substitutes were even found for halons, previously thought to be the most difficult to replace. Great Lakes Chemical Corporation of Indiana, for instance, reported that FM-100 has the same fire suppression ability as halons but with an ozone depletion potential of only 1.1 compared to 10 to 13 for halons (Wall Street Journal 1991a). 23
In many cases, new processes were more important than new chemicals. New recycling methods were developed for the refrigeration and air conditioning industry. The international fire protection community, with its long-standing concern for human health and safety, became very active in finding ways to recycle and redeploy halons. In some cases, the politics of ozone protection made for strange bedfellows. At a conference sponsored by NATO, the U.S. Air Force, and the EPA, participants agreed that essential military and civilian uses of halon were small and could be served by existing stocks if "halon banks" could be established (U.S. Air Force, EPA, and NATO 1991). 24
Progress on substitutes and alternatives, however, was determined more by innovative arrangements for information-sharing than by major technological breakthroughs. Two industry research consortia, PAFT and AFEAS, have already been discussed. With the help of the EPA, the Industry Cooperative for Ozone Layer Protection (ICOLP) was also established to make available nonproprietary, CFC-alternative information through technology-transfer workshops, an electronic database, and guidebooks. The ICOLP database, OZONET, has been linked to UNEP's International Cleaner Production Information Clearinghouse (ICPIC) in Paris. UNEP also established the OzonAction Information Clearinghouse (OAIC), with an electronic mail capability for users to exchange information, as well as data bases on alternative technologies, equipment, and product suppliers; expert contacts; country programs; corporate initiatives; and literature abstracts (OzonAction Newsletter 1991).
Some companies rose to new heights of corporate responsibility in sharing information and technology. Northern Telecom, for instance, developed a no-clean process that costs far less than and eliminates the need for CFC-113 in electronics. Digital Corporation produced an aqueous cleaning technology to replace CFC-113. In the only two instances of this sort in environmental protection, both these companies have licensed their patented technologies worldwide without a fee (interview with Stephen Anderson).
Innovative partnerships involving industry and government agencies from both developing and industrialized countries sprang up to speed the elimination of ozone-depleting substances in developing countries. The Mexican government, in partnership with the EPA, the ICOLP, and Northern Telecom, announced plans to eliminate CFCs on the same schedule as industrialized countries under the Montreal Protocol. CFC consumption in Thailand, a signatory to the Montreal Protocol, rose exponentially from 1986 to 1990; in the first four months of 1991, Thailand used 45 percent more CFCs than it did during all of 1990. Solvents constituted nearly half of this CFC use, and 97 percent of that was by Japanese and U.S. companies and joint ventures (IER 1991d). In response, several Japanese and American industry associations, along with the Thai government, the EPA and the Japanese Ministry of International Trade and Industry (MITI), announced plans to phase out solvents controlled by the protocol. In other industrialized countries as well, multinational corporations pressured their foreign subsidiaries and their host governments to reduce CFC consumption.
Meeting for the first time in September 1990, the executive committee of the Multilateral Fund designated UNEP as the fund's treasurer and established the fund's secretariat in Montreal. The most difficult task was to work out the fund's complex operational details. Unlike other programs addressing global environmental problems, the fund allocated resources before determining a process for allocation, and it placed administrative and managerial responsibility in the hands of three agencies. Establishing equitable and consistent project eligibility criteria proved to be a great challenge. The decision to fund on a concessionary basis raised thorny issues: the availability of grants and low-interest loans, for instance, could lead some countries and companies to shelve investments they had already planned to make. The fund's most straightforward task was the development of country studies to ascertain the needs and costs of individual developing countries. Within the first year, new studies were launched for seven countries (IER 1991f).
Environmentalists were critical of the fund's executive committee for being preoccupied with procedural and administrative issues during its first year of operation. The three implementing agencies seemed more concerned with their own bureaucratic interests than with coordinating their work plans to make the fund operational. As of the April 1991 meeting of the executive committee, only $2 million in contributions had been received for the 1991 budget of $50 million, and the fund had distributed no ozone-friendly technology to developing countries. Environmental NGOs and industry were disappointed when their request for observer status at executive committee meetings was denied; only those groups specifically invited would be permitted to attend (FOE 1991b:2).
Despite the early problems with the fund, the financing of technology transfers to developing countries seemed to be an easier task than had been originally envisioned. The first country studies suggested that as many as half of the initial projects would offer a positive financial return to the implementing country (IER 1991f:638). This surprising finding was a direct result of the fact that CFC substitutes and alternatives were turning out to be far cheaper and more readily available than anyone had imagined a few years earlier. Many of the industrial modifications involved cost-effective recycling or process changes that could have been implemented prior to the Montreal Protocol. Once the need, dictated by the interplay of science and policy, was clear, the cognitive impediments to change fell away, and new technologies quickly became available.
With the Third Meeting of the Parties approaching, the combination of scientific urgency and technological progress ensured that new treaty revisions would be on the agenda. Atmospheric scientists, reluctant to make any policy recommendations prior to Montreal, were now quite willing to advocate specific policies. In April, two months before the Nairobi meeting, Robert Watson told the U.S. Senate that four actions were necessary: an accelerated phaseout of controlled chemicals; worldwide compliance with the treaty; chemical recycling; and the development of not-in-kind substitutes for CFCs. In particular, Watson warned against a dependency on long-lived HCFCs and HFCs. This warning was reinforced by research by the National Oceanic and Atmospheric Administration showing that HCFC-141b has an ODP 50 percent greater than was initially estimated, making it as harmful to ozone as methyl chloroform (U.S. Senate 1991).
Unlike the First Meeting of the Parties, which generated the Helsinki Declaration prior to the London revisions, the Third Meeting of the Parties in Nairobi in June endorsed no new treaty revisions, despite the new scientific evidence. Instead, delegates at Nairobi requested other bodies to develop recommendations for adjustments and amendments to be taken up in 1992 at the fourth meeting. The parties directed a special working group to make recommendations regarding a more detailed noncompliance procedure (UNEP/OzL.Pro.3/11:15) and instructed the assessment panels to look at "the possibilities and difficulties of an earlier phaseout of the controlled substances, for example, a 1997 phaseout" (UNEP/OzL.Pro.3/11:18). They also asked the panels to suggest a possible phaseout date for HCFCs. Seven European governments, dissatisfied with the call for further study, reaffirmed their commitment to a 1997 phaseout for CFCs, halons, and carbon tetrachloride, and a 2000 phaseout for methyl chloroform.
Following the general pattern of events, scientific data soon indicated a worsening of the ozone problem, sparking new calls for stronger policies. In October 1991, the Antarctic ozone hole hit a record low (Science 1991). Shortly afterward, NASA scientists announced that ozone was thinning throughout the year, even during the summer, and everywhere except over the tropics (Science News 1991:278). Du Pont responded by announcing plans to hasten its phaseout of CFCs and halons: 1996 for CFCs and 1994 for halons (Los Angeles Times 1991).
The NASA study also cast doubt on the presumed role of CFCs in global warming, suggesting that the loss of ozone, also a greenhouse gas, could counterbalance the warming effect of CFCs, with the result that eliminating CFCs would have little or no effect on the climate (interview with Robert Watson). True to the principle that knowledge can be employed on behalf of a range of interests, this information was used by some to praise and by others to criticize U.S. policy on global warming. Those who supported the Bush administration's wait-and-see approach to the climate problem argued that the scientific uncertainty regarding the role of CFCs in global warming vindicated the administration's policy (New York Times 1991). The critics, however, presented a more complex argument. The Bush administration's "Comprehensive Strategy" lumped together all greenhouse gases and called for a freeze on emissions at 1987 levels by the year 2000. Those countries who were major producers of CFCs, with their enormous global warming potentials, could achieve their targets through the Montreal Protocol provisions and could meanwhile continue to increase their emissions of carbon dioxide, the principal greenhouse gas. The GWP of CFC-11 relative to carbon dioxide, for instance, is 4,500; for CFC-12, it is 7100 (U.S. Task Force on the Comprehensive Approach to Climate Change 1991). 25 Thus, critics declared that the new NASA study "poked a gaping hole in the administration's strategy for dealing with global warming" (Wall Street Journal 1991b). Ultimately, this argument was persuasive, and the administration was compelled to abandon its "comprehensive approach" in the negotiations on climate change.
The discovery that ozone depletion may have offset a portion of the greenhouse warming during the previous decade was only one of five major findings announced in the Scientific Assessment of Ozone Depletion, published in December 1991. There was also evidence for the first time that significant ozone losses occur in spring and summer in both the Northern and Southern Hemispheres at middle and high latitudes. In addition, the biennial modulation of the Antarctic ozone hole seemed to be giving way to a severe hole annually, accompanied by large increases in ultraviolet radiation, and losses over the Arctic, while not so enormous, had the potential to affect populated areas. Other new evidence supported the conclusion that chlorine and bromine were responsible for the ozone losses over both poles. Finally, while most of the newly calculated ODPs were similar to those in previous assessments, the new GWP results indicated that many of the GWPs reported in the Intergovernmental Panel on Climate Change (1990), especially that of methane, were likely to be incorrect (WMO/NASA 1991:xi–xiii).
Although these were the major findings outlined in the executive summary, others also had implications for policy. For instance, the new models incorporating heterogeneous processes on sulfate aerosols predicted as much as two to three times as much ozone depletion compared to models containing only gas-phase processes (WMO/NASA 1991:xiii). This finding that a major volcanic eruption could have devastating implications for the ozone layer reinforced the drive toward precautionary action. In addition, the science panel also reported that the new ODPs were larger for substances with long stratospheric lifetimes, such as the HCFCs, than for those with shorter stratospheric lifetimes, like methyl chloroform and carbon tetrachloride (ch. 6, p. 7). This finding strengthened the case for controlling HCFCs. Finally, while the science panel found that methyl bromide was the principal source of stratospheric bromine, a far more potent destroyer of ozone than chlorine, its anthropogenic sources were uncertain. Nonetheless, the assessment noted that each 10 percent reduction in methyl bromide emissions would be comparable to a three-year acceleration of the CFC phaseout schedule (xvii). The methyl bromide issue would turn out to be one of the major controversies of the 1992 treaty review process.
While they did not make specific policy recommendations, scientists in 1991 were willing to offer general advice. As in the 1989 assessment, that advice was framed in terms of limiting atmospheric chlorine loading. Even with full compliance under the amended protocol, the 1991 abundance of chlorine (3.3 to 3.5 ppb) was expected to rise to 4.1 ppb by the end of the century, thereby greatly increasing the risk of ozone loss over the middle and high latitudes. Reducing that risk, the assessment concluded, "requires further limitations on the emissions of chlorine- and bromine-containing compounds" (WMO/NASA 1991:xvii). Like the 1989 Synthesis Report, the 1991 assessment provided scenarios for limiting chlorine concentrations, although in a table rather than the
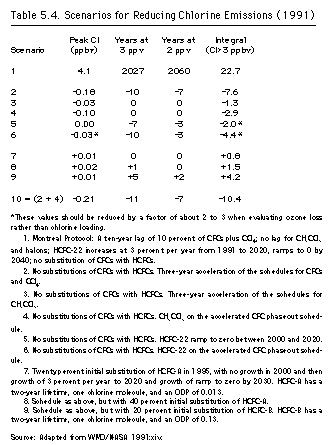
|
In many ways, as they did in 1989, the scenarios set the agenda for the policy debates. But in 1991, the range of scenarios presented was more limited. In particular, the only phaseout date considered for CFCs and carbon tetrachloride was 1997. Environmentalists criticized the assessment for failing to consider earlier dates, particularly in light of the fact that some governments had already adopted stricter timetables (interview with Liz Cook).
As in 1989, the environmental effects panel report was far less comprehensive than the scientific assessment. In fact, there was so little new information in 1991 that the panel only published an update, which included summaries for each of the 1989 chapters, rather than a full assessment. Introducing the update, the panel chairs admitted that progress had been "disappointing" and pointed to the fact that funding for research on the consequences of increased ultraviolet radiation was meager, "typically less than 1 percent of what is made available for atmospheric research in relation to ozone depletion" (UNEP 1991a:i).
Still, some new information was reported. Perhaps most important was the conclusive link between immunosuppression in humans and UV-B radiation, among dark-skinned as well as light-skinned populations (UNEP 1991a:iii). Moreover, in addition to skin cancer, exposure to sunlight was also associated with cancer of the salivary gland, suggesting the possibility of a systemic effect of UV-B in humans since the salivary gland is rarely exposed (15). Research on aquatic ecosystems focused on the effects of UV-B on phytoplankton, particularly in the waters under the Antarctic ozone hole. Phytoplankton are essential to the productivity of marine biomass, the source of more than 30 percent of the animal protein consumed by humans. New research found that targets other than DNA in photoplankton, including intrinsic proteins of the photoreceptor and photosynthetic apparatus, absorb UV-B radiation. Most importantly, a decrease in phytoplankton populations could alter cloud patterns and, concomitantly, the global climate. A hypothetical loss of 10 percent of phytoplankton would reduce the annual oceanic uptake of carbon dioxide by about 5 gigatons, an amount equal to the annual emissions of carbon dioxide from fossil fuel consumption. Thus, like the science assessment, the environmental assessment was compelled to consider climate change issues.
Despite these important new findings on phytoplankton and the human immune system, the environmental assessment was rarely cited during the ensuing policy discussions. As had been the case historically, atmospheric research set the terms of policy discourse - even though ultraviolet radiation, not ozone depletion per se, is the real threat to life on the planet. Perhaps this choice of emphasis reflects the tacit knowledge (Polanyi 1958) embedded in precautionary discourse: "Don't fool with Mother Nature." Perhaps it was enough to know that major ozone losses were occurring, whatever might be the effects. Or perhaps the lack of attention to, as well as funding for, health and environmental effects reflects the wider society's hierarchy of the sciences, under which those scientific disciplines furthest removed from everyday human life, especially physics, are frequently considered the most prestigious and are therefore the best-funded. Either way, the dynamics underlying both the production and the reception of the environmental assessment were very much a product of social factors.
Predictably, technology continued to be a driving force of the treaty revision process, second only to atmospheric science. The 1991 technology and economics assessment, involving 240 experts on six committees, included a great deal of new information uncovered since in the 1989 assessment. 26 Since 1989, technological advances had made early reductions possible, largely because industry and consumers were reducing their dependence on ozone-depleting substances more quickly than had been anticipated at the London meeting. So impressive was the progress that, by 1992, CFC consumption would be reduced to 50 percent of 1986 levels - a three-year advance on the requirements of the Amended Protocol (UNEP 1991b:ES-2). Many of the ingredients of this rapid progress, such as new processes, partnerships involving industries and governments, information-sharing arrangements, and the halon bank, have been outlined above and are acknowledged in the 1991 assessment. Thus, the Economic Options Committee found that "technological optimism" was justified because "problems which were regarded as big and difficult not so long ago have been successfully dealt with much more quickly and a lower cost than expected" (UNEP 1991c:13).
The 1991 technology assessment concluded that it was technically feasible to eliminate virtually all consumption 27 of CFCs, halons, and carbon tetrachloride between 1995 and 1997 and of methyl chloroform between 1995 and 2000. These technically feasible phaseouts are depicted in
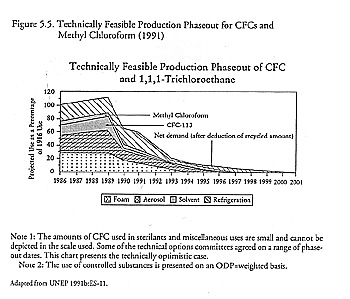
|
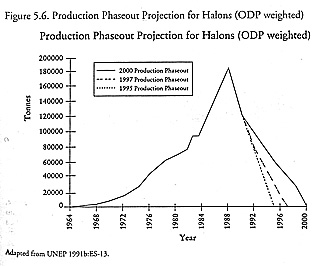
|
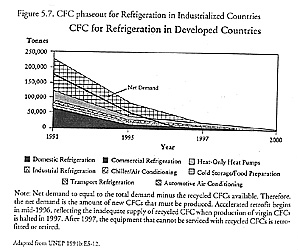
|
Knowledge production and employment were key to all aspects of the technology assessment. To a greater extent than the other panels, this panel attracted enthusiastic participation in its committees because the expert networks generated useful information for the participants themselves. In fact, the panel's research helped to speed technical progress by fostering information exchange and ongoing relationships among industry representatives. The panel also emphasized the primacy of information-sharing arrangements in advancing the phaseout dates for ozone-depleting substances, such as industry consortia for researching new substitute compounds, information sources like UNEP's clearinghouse and trade association data banks, and ad hoc partnerships among industries and government agencies. Because public awareness of the ozone issue was also important, the panel promoted the establishment of new "knowledge centers" especially in developing countries (UNEP 1991b:6–11).
Thus, the drive toward stronger precautionary action that began in the aftermath of the Montreal Protocol continued with the 1991 assessments. Both the science and the technology reports adopted 1997 as the hypothetical phaseout date for CFCs and carbon tetrachloride, and this date initially seemed likely to be incorporated into the amended treaty. The combination of urgent scientific findings and technological optimism assured that new treaty amendments would be adopted in 1992. But whether those revisions would suffice to keep pace with the rapidly deteriorating ozone layer was not at all clear.
In the United States, environmentalists were skeptical. In December 1991, the NRDC and other groups filed a petition to compel the U.S. government to honor its commitments under the amended Clean Air Act and speed the phaseout of ozone-depleting substances. The NRDC petition cited the German phaseout date of 1995 and also demanded that the EPA phase out methyl bromide. Under the U.S. law, chemicals with an ODP over 0.2 are required to be listed as Class I substances and must be eliminated; methyl bromide has an ODP of 0.7 (FOE et al. 1992:26). While seemingly confrontational, the NRDC petition was actually negotiated with the EPA; in the aftermath of the Montreal Protocol, the agency and environmentalist organizations had developed a cooperative relationship (interview with Eileen Claussen). As in 1986, domestic processes involving the Clean Air Act had foreign policy implications; in both cases, an NRDC petition spurred the United States to adopt a more far-reaching ozone policy internationally.
The environmentalist position was bolstered by new scientific findings during the winter and spring of 1992. In February scientists, reporting levels of chlorine monoxide over New England and Canada higher than those found during flights into the Antarctic ozone hole, announced that an ozone hole was likely to form over populated regions of the northern hemisphere. The scientists attributed the high levels of chlorine monoxide to sulfur particles from industrial pollution and the Mount Pinatubo eruption (Science News 1992a). Although NASA's early release of preliminary results of satellite measurements was criticized by some scientists and media sources for "setting science policy via press releases," (Wall Street Journal 1992), it prompted a quick policy response. President Bush announced that the United States would phase out production of CFCs, halons, methyl chloroform, and carbon tetrachloride, except for "essential uses," by December 31, 1995. The USA would also reexamine the phaseout schedule for HCFCs, to be banned in 2030 under the Clean Air Act, and explore a phaseout of methyl bromide (The White House 1992). Shortly afterward, legislation was introduced in the EC, and later approved, to reduce CFC production by 85 percent in 1993 and altogether by the end of 1995. In addition, Denmark, Germany, and the Netherlands were considering a phaseout date as early as 1993 (European Report 1992). 28
Environmentalists' demand for stricter controls on HCFCs was reinforced by new scientific research indicating that the chemicals release their chlorine much faster than had been thought, thereby posing a greater threat to the ozone layer. During the period when chlorine concentrations will be at their highest levels, HCFCs will be three to five times more destructive than previously foreseen. HCFC-22, a common refrigerant whose production had increased by 9 percent annually since 1986, was revealed to be 75 percent less depleting than CFCs, not 95 percent as originally estimated (Washington Post 1992).
At the April 1992 meeting of the Open-Ended Working Group, the conclusions of the assessment panels were reviewed, and proposals were developed for further treaty amendments and adjustments. These proposals included an advance in the phaseout schedule of controlled substances from the year 2000 to 1995 or 1996 and the listing of HCFCs and methyl bromide as controlled substances. Some developing countries proposed that they should only assume further obligations under the protocol after a review in 1995 (UNEP/OzL.Pro.WG.1/6/3). Greenpeace and Friends of the Earth presented a joint statement to the gathering, stating that "unlike the situation on many difficult policy questions, you are in the fortunate position to have all the science you need to take effective decisions." The statement outlined the major scientific findings and framed the parties' responsibility in terms of their legal commitment in the Vienna Convention to take precautionary action (Hohnen 1992).
For the first time since 1987, a major scientific controversy became the focus of a policy debate. One of the greatest uncertainties in the 1991 science assessment was the extent to which methyl bromide posed a threat to the ozone layer. The chemical is used as a preplanting, postharvesting, and structural fumigant. While the panel found methyl bromide to be the principal source of stratospheric bromine, each molecule of which is 30 to 120 times more effective at destroying ozone than is chlorine, it made no recommendations on methyl bromide because the reasons for the chemical's destructiveness were not well understood (WMO/NASA 1991:xii). Because very little of it reaches the stratosphere and its atmospheric lifetime is less than two years, it had been ignored previously. Environmentalists, however, insisted that the compound should no longer be overlooked.
At the April 1992 meeting, the Open-Ended Working Group, hoping to agree on a set of proposed treaty amendments to be presented at the Fourth Meeting of the Parties in the fall, could not agree on methyl bromide controls. The United States, under domestic pressure to enforce the Clean Air Act, proposed a phaseout by the year 2000; other industrialized countries favored only a freeze. Mostafa Tolba proposed a 20 percent reduction by the year 2000. Israel, however, with support from some developing countries, called for further study. Mexico went beyond most developing countries, calling for a freeze in 1995 (interview with Paul Horowitz). The working group requested a special study on methyl bromide, which was published in June and incorporated new findings presented at the international Methyl Bromide Science Workshop.
The special assessment was hastily put together; within a matter of months, the methyl bromide issue had emerged from obscurity to become perhaps the most controversial dimension of the ozone issue. There were enough uncertainties about methyl bromide to allow parties to frame the available knowledge according to their perceived interests. Yet, although the study found uncertainties, it indicated that methyl bromide could account for one-sixth of the predicted ozone loss by the year 2000 if emissions continued to grow at nearly 6 percent per year. The primary implication for policy formulation, the report concluded, was that elimination of anthropogenic methyl bromide could contribute as much to restore the ozone layer as would a three-year advance in the CFC and carbon tetrachloride phaseout schedule (UNEP 1992a:3).
Methyl bromide, the most widely used nonpetroleum pesticide after petroleum, is produced in eight countries, with industrialized countries consuming 80 percent of all production. The United States, which requires fumigation for many purposes, is the largest user, consuming 43 percent of global production. In the USA, methyl bromide is manufactured by Great Lakes Chemical Corporation and Ethyl Corporation; combined, their output is half of all global production (FOE 1992c:3). Yet, because of the Clear Air Act and domestic pressure, the world's top producing country was placed in the anomalous position of being the strongest supporter of strict controls. As in the Montreal Protocol negotiations, the United States wanted a "level playing field" (interview with Paul Horowitz). By contrast, the EC, despite its progressive CFC legislation, proved to be the strongest opponent of methyl bromide controls. Israel, the world's second largest producer and agricultural user, garnered support for its position from developing countries receiving aid, even though these countries used very little of the compound.
For the first time since Montreal, developing countries framed their political position in terms of scientific knowledge. They were joined by Israel and the Mediterranean countries within the EC. Drawing upon the uncertainties in the special assessment, critics of methyl bromide controls constructed three arguments. First, most methyl bromide is from natural sources - as much as 75 percent (UNEP 1992a:6); therefore the relatively small amount emitted from anthropogenic sources militated against controls. This argument is the weakest since only human sources of methyl bromide can be controlled. Second, an unknown quantity of methyl bromide is removed by soils and vegetation. This argument, too, is weak because the special assessment, seeking to make a conservative estimate, assumed that a large amount was deposited on soils and vegetation (7). As little as 3 to 5 percent of methyl bromide actually makes its way up to the stratosphere (FOE et al. 1992:5). The argument with the greatest credibility was that the oceans could provide a major sink for methyl bromide emissions (7). Yet the scientists also took this consideration into account and concluded that there was still cause for concern. Nonetheless, even though more was known about methyl bromide in 1992 than about halons in 1987, the scientific uncertainties were great enough to supply ammunition to those who opposed controls on methyl bromide (interview with Stephen Seidel).
The methyl bromide issue bears some similarity to the question of methyl chloroform during the first treaty review. Both problems were studied hastily, and both brought a new set of actors into the debates. Many methyl bromide producers had some experience with the ozone issue since they also produced halons, the other bromated ozone-depleting substance. But never before had the agricultural sector been involved. That involvement entailed a host of new considerations, and although there was much resistance to controls, there were also incentives for users to reduce their dependence on methyl bromide. Farmers, for instance, are at a higher risk than the rest of the population from the consequences of increased UV-B radiation. Not only are they more susceptible to skin cancer because they work outside, their crops are also vulnerable. Perhaps the strongest incentive, however, is methyl bromide's extreme toxicity; the EPA has classified it as a Category I acute toxin, the most deadly category of substances (EPA 1986c).
Environmentalists active on the ozone issue were able to coalesce with those involved in toxics, pesticides, and rural issues. In November 1992, in time for the Fourth Meeting of the Parties in Copenhagen, a coalition of seven environmental groups published a study of methyl bromide. They also launched a campaign to persuade users and producers to phase out consumption and production within the next five years. The study framed its conclusions in terms of the scientific rationale and the technological feasibility of regulative action. Emphasizing methyl bromide's high ODP and short atmospheric lifetime, the report framed the available knowledge in a powerful manner: "Over the next 20 years," it stated, "every kilogram of methyl bromide that is released into the atmosphere will contribute far more to ozone depletion than a kilogram of a better known ozone destroyer such as CFC-11" (FOE et al. 1992:1). Recognizing that action would not result from scientific information alone, the report also stressed technical information suggesting the feasibility of a phaseout. Six case studies were included: the Netherlands ban, two phaseouts in agriculture, two in grain storage, and one in residential fumigation. Citing the history of controls on ozone-depleting substances, the report argued that substitutes were constantly being found for chemicals that industry had claimed only a few year earlier were irreplaceable (16–23).
Controlling methyl bromide was one of the three major issues facing the delegates as they met in Copenhagen in November 1992, the other two being control measures for HCFCs and the structure of the Montreal Multilateral Fund. By the time of the preparatory meeting for the Fourth Meeting of the Parties, representatives had a history of working together and a certain clubbish atmosphere prevailed. No major battles were anticipated (interview with Eileen Claussen).
Agreement on the phaseout schedules for controlled substances was relatively easy. Representatives from sixty-five countries at the preparatory meeting agreed to eliminate CFCs, carbon tetrachloride, and methyl chloroform by 1996 and halons by 1994. As usual, Mostafa Tolba circulated his own personal proposal on the most contentious issues, suggesting a freeze of methyl bromide production and consumption in 1995 at 1991 levels, a 25 percent cut by 2000, and a scientific study of the chemical to determine future control measures. Tolba also proposed a freeze on HCFC consumption by the end of 1995, a cut of 25 percent by 2000, of 50 percent by 2010, and a full phaseout by 2020. Last, Tolba suggested that the Interim Multilateral Fund be transformed into the Montreal Multilateral Fund, as called for at the London Conference two years before, and that $500 million be allocated for the years 1994 through 1996 (UNEP 1992a). All three proposals were accepted at the preparatory meeting and presented to the ministerial meeting the following week (UNEP/ OzL.Pro.4/Prep/2).
Meeting from November 21 through 23, the ministers and high-level officials from seventy-four countries and the EC agreed to Tolba's proposed phaseout schedules for CFCs, carbon tetrachloride, methyl chloroform and halons. They also agreed to phase out all but essential uses of HCFCs by 2020, with interim reductions beginning in 2004. Perceiving a need for extended HCFC use in air conditioners for large buildings, the United States argued successfully for a total phaseout in 2030. On the contentious issue of methyl bromide, Israel and the Mediterranean countries in the EC blocked agreement on reductions, despite support for such measures from all other industrial countries. Instead, the parties agreed to freeze production and consumption at 1991 levels by 1995 for Article 2 countries, with no restrictions on use by Article 5 countries. The decision was accompanied by a nonbinding resolution calling for an evaluation of methyl bromide within two years, with a possible phaseout by the year 2000. 29 For a summary of the Copenhagen revisions, see table 5.5.
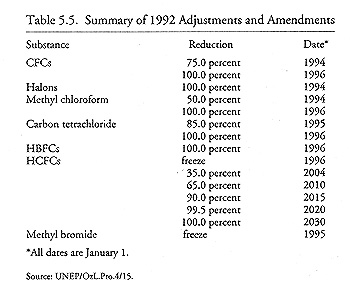
|
The Copenhagen meeting also addressed the financial mechanism. Contributions to the fund for 1991 and 1992 had come late from many contributors; some parties, including Eastern European countries, had financial difficulties, and others, such as France and the U.K., had the wherewithal but did not meet their obligations. Moreover, developing countries, frustrated with the attempt by some parties to alter the structure of the Multilateral Fund, had walked out of a meeting of the Open-Ended Working Group in July 1992 (UNEP/OzL.Pro.4/15:2). Yet, on the positive side, developing countries that doubted in 1989 whether they could achieve 50 percent reductions in CFC use by 2010 were now planning to eliminate the chemicals entirely by the year 2000. This transformation was made possible by information sharing and the Interim Multilateral Fund (Tolba 1992:3). Contrary to the expectations of many and despite the financial shortfalls, representatives from industrialized and developing countries on the fund's executive committee were working together harmoniously and efficiently and had adopted all decisions by consensus (UNEP/OzL.Pro.4/15:6).
The Copenhagen meeting agreed to establish the Multilateral Fund on a permanent basis, to operate in 1993 with a $113 million budget for that year and $340 to $500 million for 1994 through 1996. The Open-Ended Working Group was requested to advise the Fifth Meeting of the Parties at the end of 1993 about the exact size of the fund from 1994 through 1996. It was also decided that the executive committee should operate under the procedures and guidelines formulated for the Interim Multilateral Fund. The temporary difficulties of Hungary, Bulgaria, and Poland in making their contributions in convertible currency were noted, and those parties were urged to explore in-kind contributions (UNEP/OzL.Pro.4/ 15:22).
Although article 8 of the Montreal Protocol states that the parties shall establish procedures and institutional mechanisms for determining noncompliance, the London revisions had skirted the issue. Consequently, the parties at the Copenhagen meeting adopted a noncompliance procedure and created an implementation committee consisting of ten parties that are to meet twice a year. Reservations regarding a party's implementation of its treaty obligations are to be submitted to the secretariat, which gathers information about the matter and may refer it to the implementation committee, which in turn seeks an amicable solution and may refer the matter to the Meeting of the Parties. According to the situation, the noncomplying party may be given appropriate assistance to facilitate compliance, or it may receive a warning, or it may be suspended from its rights and privileges under the protocol (annexes IV and V, UNEP/OzL./Pro.4/15:46–47).
The Copenhagen Revisions received a mixed response. UNEP's own press release, while lauding the amendment process as "an environmental success story," suggested that the new control measures were not sufficiently stringent. On the one hand, the measures represent "the strongest package of global environmental law ever enacted." On the other hand, according to Mostafa Tolba, "the question remains, however: is this enough? We are in the hands of scientists. From them - and we have sought advice from the best in the world - we know that the answer is 'No.' This package is not enough. We have made progress, but we have far to go" (UNEP 1992b).
Thus, even UNEP, with a clear bureaucratic interest in reveling in its own success, had to admit that the agreement was based more on political and economic considerations than on the scientific requirements for saving the ozone layer.
Environmental organizations were even more forthright in expressing their misgivings about the Fourth Meeting of the Parties. They were especially critical of the parties for failing to reduce methyl bromide emissions, pointing out that the parties' own scientific advisory panel had concluded that methyl bromide reductions would bring substantial near-term benefits to the ozone layer. Environmentalists also complained that the agreement: (1) sanctioned a further thirty-eight years of HCFC use, despite the widespread availability of safer CFC substitutes; (2) aligned the phaseout dates for CFCs and halons nearer to the schedules of the chemical industry than the requirements of the ozone layer; and (3) allowed essential-use exemptions for most chemicals, creating a loophole that could allow continued production indefinitely. Citing a pattern of events that had begun in Montreal, environmentalists recalled that each agreement had been rapidly outstripped by accelerating ozone destruction. The Copenhagen accord, they predicted, would be no exception (FOE 1992b).
Clearly, the Copenhagen Revisions served further to institutionalize the discourse of precautionary action on ozone, first articulated by the international community in 1985 with the Vienna Convention and continued with the Montreal Protocol and the London Revisions. Yet, rather than committing themselves to a full ounce of prevention, which might have been worth a pound of cure, the Montreal Protocol parties, in Copenhagen just as in London two years before, opted for only half an ounce of prevention. Consequently, while the new control measures represented progress, they did not represent a solution to the problem.
Within five years of the signing of the Montreal Protocol, the treaty had already been revised twice as policymakers attempted to keep pace with scientific and technological developments. Clearly, from the 1974 discovery of the CFC-ozone link to the 1992 treaty revisions, ozone policy was knowledge-driven. Yet science and technology did not provide a set of objective facts from which policy could be deduced. Rather, specific modes of framing and interpreting the available knowledge were incorporated into policy discourses. Even after Montreal, when the range of scientific uncertainty had greatly narrowed, rhetorical and discursive strategies continued to be integral elements of the policy milieu. With the emergence of greater scientific certainty and the discovery of indisputable evidence of CFC-induced ozone depletion, policy action could no longer properly be considered precautionary. The new goal, therefore, was to stem the tide of environmental deterioration and to limit damage to the ozone layer rather than to prevent any harm whatsoever from occurring. But the rapidly deteriorating ozone layer - over Antarctica, over the Arctic, and globally - suggests that precautionary action was implemented inadequately and belatedly.
Many of the discursive strategies employed during the Montreal Protocol negotiations were also prevalent during the treaty revision process. First, the chlorine-loading approach, which was implicit in the original treaty negotiations, became the predominant mode of framing scientific information after Montreal. Other approaches, including computer-generated models and ozone depletion potentials, were also employed, but policy goals were always framed in terms of reducing atmospheric chlorine concentrations. Moreover, as had been the case historically, ozone discourse continued to be dominated by the atmospheric sciences, and the health and environmental consequences of ultraviolet radiation received almost no attention.
Second, even during the Montreal Protocol negotiations, the perceived availability of chemical substitutes and alternatives was more a matter of political and economic factors than of science and technology. After Montreal, those political and economic factors generated a major psychological shift among both producer and user industries, resulting in an astonishing rate of technological progress. The rapid developments engendered a sense of optimism in policy circles: not only must ozone-depleting chemicals be strictly controlled, they could be. Thus, scientific and technological discourse combined to effect swift and decisive action.
Third, developing countries became key participants after Montreal. Although many developing countries had strong misgivings about the treaty, they never voiced their reservations in scientific terms. While they framed their concerns in terms of equity and sovereignty, their compliance with the treaty was determined largely by the availability of technical information. The controversial question of the financial mechanism was ultimately a matter of redistributing knowledge from the information-rich North to the information-poor South. Both sides had much to gain from the arrangement. The North was most vulnerable to the effects of ozone depletion and could not expect the South to sacrifice its economic welfare to rectify a problem caused by its own consumption habits. The South also embraced the discourse of damage limitation and had a perceived interest in saving the ozone layer, especially if it could do so without hurting itself economically. Despite the nonprecedential language demanded by the United States, the Multilateral Fund, which is at root an information-sharing mechanism, does indeed establish a precedent for future environmental agreements.
One may view the Montreal Protocol and its revisions in either a positive or a negative light. On the one hand, the revised treaty represented "the strongest package of environmental law ever enacted." In 1987, the parties reluctantly agreed to a 50 percent reduction of CFCs by the year 2000; in 1992, with little debate, they decided to eliminate the chemical by 1996. Other aspects of the treaty were equally progressive. On the other hand, environmentalists were correct in claiming that the revisions did not go far enough. Part of the problem is that, because of the accumulation of the long-lived CFCs in the atmosphere, little could be done at such a late date to bring peak chlorine concentrations below 4 ppb. Yet environmentalists rightly claim that the parties did not do everything possible to protect the earth's ozone layer. While future levels of damage were limited, the precautionary principle was not applied rigorously.
Note 1: Partially halogenated halocarbons contain hydrogen, which causes them to break down more rapidly in the lower atmosphere. Their ozone depletion potentials are consequently much smaller than those of the fully halogenated compounds regulated by the Montreal Protocol. While HCFCs still pose some risk because they contain toxic chlorine, HFCs (hydrofluorocarbons) contain no chlorine and so do not affect the ozone layer. HCFCs and HFCs, like CFCs, are also greenhouse gases. Back.
Note 2: In the long run, althoughU.S. industry complained profusely about it, the U.S. aerosol ban helped it CFC producers by inducing them to develop substitute compounds and technologies. Because the United States had already eliminated aerosol uses, it had greater difficulties meeting the Montreal Protocol requirements and was forced to take the lead in developing CFC substitutes for other uses. Back.
Note 3: Interestingly, the FOE publication bases its conclusions on the economic data found in Forest Reinhardt's Harvard Business School case study, cited above, to make its case. Again, information can be interpreted according to subjective orientations, and the same data can lead to very different conclusions. Back.
Note 4: Note that, 2 percent of profits represents the same amount as the $600 million cited in the Harvard Business School case study, although the two numbers make very different impressions. Back.
Note 5: It is noteworthy that an 85 percent cutback, which was necessary to stabilize CFC concentrations, was originally proposed by John Hoffman of the EPA back in 1986. Once the Antarctic ozone hole had been conclusively linked to CFCs by the Ozone Trends Panel, the goal of stabilizing concentrations took on new urgency. Back.
Note 6: The four assessment panels effectively replaced UNEP's Coordinating Committee on the Ozone Layer (CCOL), which had been providing scientific information to participants since the Vienna Convention negotiations. Back.
Note 7: France's leadership role on the global climate change issue, which contrasts sharply with its sluggishness on the ozone problem, may be related to its heavy dependence on nuclear power for its energy needs. With less reliance on fossil fuels, France may be said to enjoy a comparative advantage in international efforts to combat greenhouse gas emissions. Back.
Note 8: One reason for the large attendance may have been that ICI, anticipating further domestic controls on CFCs, realized that they could only export to developing countries if those countries were parties to the agreement. In an ironic effort to promote widespread ratification of the treaty it had opposed, ICI paid the travel expenses for some delegates from developing countries to attend the London conference (interview with Mike Harris). Back.
Note 9: The main natural source of atmospheric chlorine is methyl chloride (CH3Cl) from the oceans (interview with Robert Watson). Back.
Note 10: The same rationale has been used in the global climate change debates to support controls on methane emissions. With its atmospheric lifetime of 10 years, as compared to 120 for carbon dioxide, the benefits of methane controls could be felt relatively soon (UNEP/OzL.Pro.WG.II(1)/4:18). Back.
Note 11: There was still the occasional skeptic, but these were iconoclasts and generally driven by an ideological motivation (see, for instance, Wall Street Journal 1989). Back.
Note 12: Japan's primary objection to controlling methyl chloroform was that its fire laws did not allow it to use alcohol-based solvents. Eventually, Japan's fire laws were rewritten (interview with Stephen O. Anderson). Back.
Note 13: The isolation of the Soviet Union's chemical industry was also evident in the fact that it did not produce methyl chloroform. Thus, the Soviet Union was placed in the anomalous position of promoting a ban on methyl chloroform while leading on nuclear power fthe fight to save carbon tetrachloride. Back.
Note 14: India, along with China, had not yet signed the Montreal Protocol at the time of this statement. Back.
Note 15: The results of the British, Indian, and U.S. studies, along with those of another British report, were reviewed and synthesized in a report that concurred with these estimates, which was presented at the May 1990 funding meeting in Geneva (Markandya and Pargal 1990). Back.
Note 16: Most countries sent different delegates to the two groups, which caused problems because delegates in one group did not know what was happening on the other (interview with Eileen Claussen). Back.
Note 17: Although EPA administrator Lee Thomas had pledged as early as September 1988 to eliminate CFCs, that election-year statement was never adopted as an official policy. Back.
Note 18: Note that these are the same EC countries that promoted a strong Montreal Protocol during negotiations four year earlier. Back.
Note 19: The thirteen governments were Australia, Austria, Belgium, Canada, Denmark, the Federal Republic of Germany, Finland, Lichtenstein, the Netherlands, New Zealand, Norway, Sweden, and Switzerland. The declaration was appended to the treaty revisions as a separate statement. Back.
Note 20: For some U.S. participants, the EC's support for a later phaseout of methyl chloroform cast some doubt on its commitment to strong action on CFCs (interview with Stephen Seidel). Back.
Note 21: Environmentalists, already distrustful of the funding mechanism because of the central role of the World Bank, grew even more skeptical when the executive committee barred nongovernmental observers from its meetings (interview with Liz Cook). Back.
Note 22: For halons, the new EC regulation called for a 50 percent cut by 1995 and a complete phaseout by the year 2000. For carbon tetrachloride, the regulation called for a 50 percent reduction by 1992, 85 percent by 1995, and a phaseout by 1998. As in the London Revisions, methyl chloroform would be banned by 2005. Back.
Note 23: Other companies expressed skepticism about the long-term commercial viability of any substitute compounds containing bromine. Du Pont, for instance, would not invest in bromated chemicals, believing that they were likely to be phased out in the next round of treaty revisions. Back.
Note 24: In November 1991, however, the U.S. Government Accounting Office published a report saying that, although the Pentagon had taken important steps to phase out the CFCs, the work was proceeding slowly. The GAO investigators found that the Navy was continuing to purchase products that used CFCs and halons (New York Times 1992a). Back.
Note 25: essence, the plan, formulated by knowledge brokers associated with the Department of Energy, merely renamed the reductions mandated by the Montreal Protocol as a climate stabilization strategy. Ironically, the Department of Energy was one of the U.S. agencies that challenged the strong position put forth by the State Department and EPA during the Montreal Protocol negotiations. Back.
Note 26: In response to complaints and mishaps resulting from the chemical industry's virtual exclusion from the 1989 technology assessment panel, more representatives from producer industries were included in 1991. Back.
Note 27: Recall that consumption is defined by the Montreal Protocol as production plus imports minus exports. Back.
Note 28: Japan, under pressure from the EC and the USA, also announced that it would ban production of CFCs, halons, and carbon tetrachloride by the end of 1995 (Kyodo News Service 1992). Back.
Note 29: Two months after the Copenhagen meeting, William K. Reilly, in one of his final acts before leaving his position as administrator of the EPA, ordered a ban on production and importation of methyl bromide by the year 2000 (Washington Post 1993). Back.