|
|
|
|
Ozone Discourses: Science and Politics in Global Environmental Cooperation , by Karen T. Litfin
| There just wasn't scientific or economic justification to proceed. How do you trade a possible [environmental] risk for a [business] risk that is real? |
| -Joseph Steed, Du Pont environmental manager |
Certain scientific and political events of the 1970s and early 1980s, particular in the United States, spurred later developments that shaped the Montreal Protocol process. Because the United States assumed a leadership role both scientifically and politically, my discussion is not prejudiced by having the United States as a focus. Rather, this emphasis can shed some light on the distinct roles played by specific U.S. agencies, industries, environmental groups, and citizens. This chapter also surveys the evolution of the relevant scientific networks and the varying technical assessments of the ozone problem.
Consistent with the argument in the previous chapter, this chapter confirms that science defined the parameters of the political debate and that certain modes of framing the relevant knowledge had important political ramifications. For instance, the framing of the ozone problem as a human health issue rather than an ecological one conditioned the political context of the entire debate. Moreover, from the beginning, the existence of scientific uncertainty was used by all participants in the debate to support their particular positions.
A Brief Description of the Ozone Layer
Unlike the familiar diatomic compound of atmospheric oxygen, ozone is a rare variant composed of three oxygen atoms. Most atmospheric ozone is concentrated in the stratosphere, a region roughly ten to fifty kilometers above the earth's surface characterized by temperatures that generally increase with altitude (United Nations Environment Programme [UNEP] 1987b:8–9).
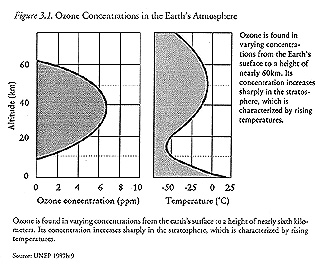
|
Solar radiation provided the conditions for the emergence of life, but earth's first organisms had to evolve under water, where they were shielded from ultraviolet radiation. Visible light penetrates water much more than does ultraviolet light, and some organisms developed the ability to photosynthesize. With the release of oxygen by oceanic plants billions of year ago, the ozone layer was created, allowing some life forms to leave the protective water and evolve on land. Without this veil, terrestrial life could not be sustained. Ozone absorbs all ultraviolet radiation with wavelengths shorter than about 290 nanometers (UV-C), most of it in the 290 to 320 nanometer range (UV-B), and little above 320 nanometer (UV-A) (Smith 1974:213). While UV-A is relatively innocuous, UV-C is lethal and UV-B is harmful to most life forms (Frederick 1986:121–23).
Ultraviolet rays with wavelengths shorter than about 240 nanometers are sufficiently energetic to dissociate diatomic oxygen molecules, which then absorb energy and heat the upper atmosphere. The single atoms of oxygen then combine with other oxygen molecules to form ozone, which in turn can be split apart by ultraviolet radiation into a single atom and diatomic oxygen again. The principal reactions can be written as follows:
O2 + UV (40–240 nm) --> O + O
O2 + O --> O3
O3 + UV (240–320 nm) --> O2 + O
Ozone is continuously created and destroyed, primarily above the tropics, and carried by winds toward the poles where its concentration is greatest. The rates of ozone formation and destruction are determined by the amount of available solar radiation, which in turn varies with latitude, seasonal variations, and the eleven-year solar cycle (Brasseur 1987:10)
Certain trace gases, such as chlorine (Cl), bromine (Br), nitric oxide (NO), and the hydroxyl radical (OH), accelerate ozone destruction. Recent increases in all of these ozone depleters can be ascribed to human activities (UNEP 1987b:11). Chlorofluorocarbons (CFCs), the primary cause of recent increases in stratospheric chlorine, remain in the atmosphere for about one hundred years (UNEP 1987b:14). This means that stabilizing atmospheric concentrations of chlorine would require not merely a freeze but drastic cuts in CFC production.
The most significant fact about the reactions of these trace gases with ozone is that they are catalytic, meaning that the ozone depleter emerges from the photochemical process unscathed, ready to destroy more ozone molecules. For example, one chlorine atom can destroy as many as one hundred thousand molecules of ozone, so that concentrations in the parts-per-billion-by-volume (ppbv) range may be dangerous (National Aeronautics and Space Administration [NASA]/World Meteorological Organization [NASA] 1986:71). A catalytic reaction cycle can be written as follows:
Cl + O3 --> ClO + O2
ClO + O --> Cl + O2
The net result is:
O + O3 + Cl --> O2 + O2 + Cl
This paints a simplistic picture of an exceedingly complex chain of reactions. Photoche mistry in the stratosphere is quite different from what we find at the earth's surface. Ordinarily stable molecules can be destroyed by the very energetic photons reaching the stratosphere. The rates of the hundreds of subsequent reactions vary greatly with temperature and pressure.
A good deal of stratospheric chlorine is neutralized by reacting with methane (CH4) to form hydrochloric acid (HCl).
CH4 + Cl --> CH3 + HCl
While some methane occurs naturally, much of it is generated from human sources such as rice paddies, cattle raising, and oil fields. The growth in atmospheric methane is expected partially to offset ozone depletion from chlorine sources. But this is a mixed blessing since methane is also a major greenhouse gas (Stordal and Isaksen 1986).
While winds transport ozone throughout the stratosphere and stratospheric ozone sometimes descends to the troposphere, ozone created in the troposphere does not reach the stratosphere, though it will absorb some ultraviolet radiation (interview with Robert Watson). This means that human sources of ozone cannot compensate for losses in the stratosphere, which is perhaps just as well, since ozone at ground level is highly poisonous. Thus, solutions to the ozone problem have entailed curtailing potential depleters rather than inventing schemes to replace what is lost.
Biological Effects of Increased Ultraviolet Radiation
Relatively little research has been done on the health and environmental consequences of ozone depletion compared to the vast quantity of work generated on the physical and chemical processes it involves. One participant in the Montreal Protocol process lamented this dichotomy, noting that "while the U.S. spends about $200 million a year on atmospheric research, the entire world spends less than $1 million on effects research" (testimony of John Hoffman: United States Congress 1987c:136). Whether because of lack of attention or the existence of substantial consensus, the effects literature has not spawned the level of debate seen in the atmospheric sciences. Nor were the biological effects a major source of political controversy during the treaty negotiations. Hence, I will simply summarize the information available to the participants in the negotiations. Three reports issued by the Environmental Protection Agency (Titus 1986; EPA 1986a; EPA 1987a) were the best sources of information available during the Montreal Protocol negotiations.
Most studies of the health effects of ozone depletion have focused on the UV-B part of the spectrum, because, as mentioned, ozone absorbs virtually all dangerous UV-C under all foreseeable scenarios, while UV-A, which ozone does affect, is relatively harmless. Each 1 percent decrease in ozone will allow 2 percent more UV-B radiation to reach the earth's surface, and this is expected to lead to a greater incidence of skin cancer, cataracts, and immune disorders among humans, as well as interfering with terrestrial and aquatic ecosystems (Frederick 1986:130).
Nonmelanoma skin cancer, specifically basal and squamous cell carcinoma, has been definitively linked to the cumulative effects of UV-B exposure (Emmett 1986:138). Every 1 percent decrease of total column ozone (i.e., the total amount of ozone in a vertical column with its base at a given point on the earth's surface) is predicted to produce a 3 percent rise in the incidence of nonmelanoma skin cancer (UNEP/OzL.Pro.WG.II[1]/ 4:7). Melanoma, a far more deadly type of skin cancer, may be associated with acute radiation exposure, but this link is more speculative (140–41). Caucasians living in the mid-latitudes are especially vulnerable to both forms. In the United States, one out of every three cancers is a skin cancer, and one in seven Americans is expected to develop this disease at some point. During the 1980s, increased skin cancer rates may have been caused by the growing popularity of outdoor activities (UNEP 1987b:28). According to EPA estimates, unrestricted CFC growth would result in 180 million new cases of skin cancer and 3.5 million cancer deaths by the end of the next century in the United States alone (:exhibits 7–3 and 7–5 in EPA 1987a). But these estimates may be too low because they are based on linear projections from 1982 data that were later found to be overly conservative (testimony of Darrell Rigel: United States Congress 1987b:70).
Although experts largely agree on their quantitative estimates of health effects from ozone depletion, they differ on their interpretations of the data. Dr. Darrell Rigel, for instance, suggests that the United States is facing an "epidemic" of skin cancer. In a letter responding to his testimony, Dr. Margaret Kripke, who chaired the EPA's Scientific Advisory Board on ozone, questions Rigel's use of the term "epidemic." In her view, the term is "alarmist and conjures up pictures of bubonic plague" (United States Congress 1987b:617). Kripke, herself a cancer specialist, believes that the skin cancer issue has been overblown and that the more serious problems are those involving the human immune system, global food supply, and ecosystems (1989:3; interview).
EPA scenarios also predict that an additional 2.8 million Americans born before 2075 will be afflicted with cataracts, a clouding of the lens that blurs vision (exhibit 7–21 in EPA 1987a:7–26). Each 1 percent total column ozone depletion is expected to lead to a worldwide increase of 100,000 blind persons (UNEP/OzL.Pro.WG.II[1]/4:7). While cataracts are routinely removed by surgery in the industrial world, people in the developing world are unlikely to have access to the operation.
Medical researchers believe that UV-B interferes with the human immune system, lowering the body's resistance to infectious diseases. The few studies in this area have found a link between UV-B and viruses such as leishmaniasis and herpes (Titus 1986:6). Higher UV-B levels might also decrease the effectiveness of some inoculation programs; patients who do not build immune responses to the antigens might develop the disease itself (Shea 1988:15).
Laboratory experiments on two hundred different plant species, including most major crop species, indicate that about two-thirds of them are sensitive to higher levels of UV-B. Increased radiation can damage plant hormones and chlorophyll, leading to retarded growth. Experiments on plants were performed holding temperature and precipitation constant, but if predictions of climatic change are correct, such stability cannot be assumed (Teramura 1986). Some species also exhibit altered chemical composition that could affect their nutritional value (UNEP/ OzL.Pro.WG.II[1]/4:7).
The effect of ozone depletion on marine life could also be harmful to humans; fish account for 18 percent of the animal protein that people consume worldwide, and 40 percent in Asia (Titus and Seidel 1986:7). Because it can penetrate to a substantial depth in clear water, UV-B radiation poses a particular threat to the single-celled algae called phytoplankton that live near the water's surface. These algae are at the bottom of the aquatic food chain, and most fish depend upon them somehow for their survival. It is possible that they could move to a deeper level to avoid the increased radiation, but this would decrease their access to the photosynthetically active radiation on which they depend. Other species would also be affected by increases in UV-B, especially those whose larvae hatch near the water's surface; many of these species are already exposed to as much UV-B as they can tolerate (Worrest 1986).
Other effects of ozone depletion include degradation of polymers and increased urban smog. Ultraviolet radiation is known to stimulate the formation of ground level oxidants (smog), a process likely to escalate with greenhouse warming. One study of the damage to polyvinyl chloride (PVC) suggests that for a 26% depletion of the ozone layer by 2075, the undiscounted costs in the United States would be $4.7 billion in 1984 dollars (Titus and Seidel 1986:7).
A Brief History of the Fluorocarbon Industry
At the 1930 annual meeting of the American Chemical Society, Thomas Midgely stood in front of his fellow scientists, inhaled the vapors from a cup of clear liquid, and blew out a candle's flame. His demonstration was meant to show that the chemical was neither flammable nor highly toxic (Washington Post 1988a). Midgely, who had been hired by General Motors' Frigidaire Division to invent a new refrigerator coolant, had synthesized dichlorodifluoromethane (CFC-12), the first of the miracle chlorofluorocarbons. In the process of vaporizing within refrigerator coils it absorbs heat, thereby allowing the coils to cool enough to refrigerate food. Du Pont, a cosponsor of Midgely's work, patented the process and began production under the trademark Freon. 1
CFCs are produced by reacting simple chlorinated organic compounds (chlorocarbons) with hydrofluoric acid (HF), which is made by reacting fluorospar (calcium fluoride) with sulfuric acid. Fluorospar is mined, typically by the CFC producers themselves, in various locations throughout the world. CFC-11 and -12 plants, with an average annual production capacity of fifty million pounds, generate large amounts of toxic hydro-chloric acid (HCl) which must be either sold as a chemical or disposed as a waste product.
Fully halogenated CFCs are virtually chemically inert near the earth's surface because they lack hydrogen, an element that tends to break apart the compounds and release toxic chlorine into the lower atmosphere. Five fully halogenated CFCs are commercially available: CFC-11, CFC-12, CFC-113, CFC-114, and CFC-115. HCFC-22, the most common partially halogenated CFC, is used as a coolant in large commercial refrigerators and in manufacturing certain polymers. It has replaced some of the CFCs regulated by the Montreal Protocol because it has a lower ozone depletion potential than the fully halogenated CFCs. 2
Production of CFC-11 and -12 grew rapidly, from about 1.2 million pounds in 1931 to 76 million pounds by 1950 (Chemical Manufacturers Association 1987). By 1945, most ammonia- and sulfur dioxide–based refrigerators had been replaced by ones using CFC-12. During World War II, Dow Chemical Company began using CFC-12 to produce a new kind of insulating foam under the trademark Styrofoam, causing CFC production to nearly double in the five years following the war. CFCs were also used in the war to combat malaria, functioning as propellants in cans of insecticide.
As later advocates of international regulation frequently pointed out, every decade since their invention has seen at least one major new use for these "miracle chemicals." In the 1950s, CFC-11 was used as a blowing agent in flexible polyurethane foams, which became ubiquitous in furniture, carpet pads, and automobiles. By the end of the decade, CFC-11 was also being used to blow rigid polyurethane foams, which eventually overtook fiberglass and other materials to dominate the insulation market (Washington Post 1988a:A-18).
The proliferation of air conditioning in homes and public places during the 1960s facilitated the demographic expansion of the United States into the sunbelt. Mobile air conditioners became standard equipment in American cars and trucks, eventually accounting for more CFC refrigerant consumption than all other uses combined. Since vehicles are poorly insulated and their hoses are prone to leakage, the average mobile air conditioner contains enough refrigerant to cool an entire house (testimony of George Kerckhove; U.S. Senate 1987b:499–501).
By 1974, when scientists first linked CFCs to ozone depletion, nearly two billion pounds of CFCs were being produced each year - about ten times the 1951 figure. Over half of this was for aerosol propellants, primarily for personal-care items like hair sprays and deodorants. Even when a combination of legislation and consumer aversion to aerosol propellents in the United States, Canada, and Sweden slowed global CFC production, other applications continued to grow, soon offsetting that decline (see figure 3.2).
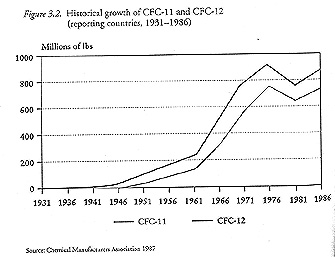
|
CFC-113 entered the market in the 1970s as a solvent and degreaser for electronic components. Because of its high cost, it was first used by the defense and aerospace industries. But when the computer industry began miniaturizing its components, a process requiring a reliable solvent to remove the tiniest particles, American and Japanese CFC-113 production skyrocketed. Annual worldwide production tripled between 1976 and 1987 (based on estimates in Hammitt et al. 1986:60).
Another important class of ozone-depleting chemicals are the halons, which contain bromine instead of chlorine. Although they are produced in much smaller quantities, they are of special concern because their ozone depletion potential per molecule may be ten times that of the CFCs. Halons were developed by the U.S. Army Corps of Engineers after World War II for extinguishing fires in tanks and aircraft. They are most useful under conditions where the use of water might damage expensive equipment or risk people's lives. The military continues to be a primary consumer of halons, followed by banks and commercial airlines. Total global production of the three halons (Halon-1211, -1300, and -2400) in 1985 was about seventeen million pounds, with the United States accounting for about half the total (exhibit 4.1 in EPA 1987a:4.3).
CFCs have other minor uses, among them sterilizing medical equipment, fast-freezing produce, puffing tobacco, and stabilizing whipped toppings. In 1987, the combination of these uses accounted for roughly forty-two million pounds yearly on a global basis (based on estimates in Hammitt et al. 1986)
As of 1986, CFCs in the United States represented approximately $1 billion in sales, or about 30 percent of the world market, by five producers: Du Pont, Allied-Signal, Pennwalt, Racon, and Kaiser Chemical. The principal competitors in overseas markets were Imperial Chemical Industries (ICI) of the United Kingdom, Farbwerke Hoechst of West Germany, and Atochem, a subsidiary of the French Elf-Aquitaine (Jachtenfuchs 1990:265). While virtually all U.S. production was consumed domestically, the EC exported nearly half of its CFCs (Chemical Manufacturers Association 1987). During the international ozone negotiations, this difference in markets may have exacerbated the tensions already present in the relations between the U.S. and European chemical industries. - particularly between Du Pont and ICI, whose mutual hostility went back nearly two hundred years to their competition in the world gunpowder cartel (Taylor 1984).
Generally, the value of CFCs relative to the products containing them is small. For instance, a $1000 refrigerator may contain merely $10 worth of CFCs; the CFCs in the foam and coolants in a $10,000 car may cost only $50. But in 1987 over ten thousand users purchased CFCs from the five U.S. producers, accounting for over $28 billion in goods and services and 253,000 jobs. Servicing of refrigerators and air conditioners adds $5.5 billion in value and 472,000 jobs. The estimated value of American-installed equipment relying on CFCs was over $135 billion (Alliance for a Responsible CFC Policy 1987:V-1).
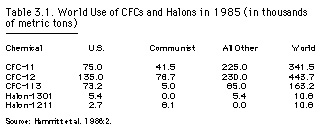
|
A Brief History of the Ozone Controversy
Initial fears about ozone depletion were unrelated to CFCs. Concern about the ozone layer first emerged in 1970, when the United States, Britain, and France were planning to build a fleet of supersonic transport airplanes (SSTs). In that year, a German scientist theorized that the oxides of nitrogen emitted in the exhaust of the high-flying planes would catalytically consume ozone (Crutzen 1970). Harold Johnston, a Canadian chemist at the University of California at Berkeley, calculated that the planned fleet of five hundred SSTs would cause a 22 percent depletion of the earth's ozone layer (Johnston 1971). Despite a prominent study study that concluded that ozone reductions from the SST would be insignificant, Congress voted in 1971 to terminate funding for the program (for a history of the SST controversy, see Horwitch 1982).
Several aspects of this controversy were important for later developments. First, this was one of the first times that scientists became involved in environmental policy. It is noteworthy that the original draft of Johnston's paper, which would be considered cautious by today's standards, was rejected by Science editors for having too many references to political questions (Dotto and Schiff 1978:65). Second, the SST controversy provided the first major opportunity for atmospheric chemists and meteorologists to spar with one another, a conflict that has abated somewhat but was still evident even after the 1985 discovery of the Antarctic ozone hole. Third, after the U.S. program was defunded and the question of landing rights for British and French SSTs became primary, the Europeans accused Americans of attempting to export their own environmental standards as a veil for their economic interests, an allegation that resounded during the Montreal Protocol process.
Perhaps the most important outcome of the SST controversy was the new interest it generated in atmospheric research. Until then, little was known about the upper atmosphere, and communication between atmospheric chemists and dynamicists was poor. In 1971, the U.S. Department of Transportation established the major Climatic Impact Assessment Program (CIAP) to study the potential impact of the SST. The program's name reflected early concerns about climate, concerns that later shifted to ozone loss once the skin cancer link gained publicity. 4 CIAP scientists concluded that SSTs would not be as harmful as originally feared (CIAP 1973). By then, a team of scientists working with Richard Stolarski and Ralph Cicerone had determined that chlorine in the exhaust of the planned space shuttle posed a more serious threat to stratospheric ozone, since it destroys ozone far more efficiently than do the nitric oxides produced by the SSTs (Stolarski and Cicerone 1974).
The space shuttle was the first man-made source of chlorine to be studied. Politically, it had important repercussions, for it sparked a great deal of concern within the National Aeronautics and Space Administration. At the time, expenditures on the shuttle constituted one-third of NASA's budget. As the successor of the Apollo project and the only man-in-space program, it was NASA's prestige project. Small wonder, then, that scientists were pressured by NASA to downplay the significance of the shuttle as a source of chlorine and to emphasize volcanoes instead (Dotto and Schiff 1978:127). NASA convinced Congress that it was the best agency to study stratospheric ozone. It quickly became the world's foremost authority on ozone, and it has remained so up to the present. The NASA budget for stratospheric research was between $15 and $20 million annually during the years of international negotiations on the ozone convention, an amount comprising approximately 70 percent of global funding on stratospheric research (interview with Robert Watson).
Meanwhile, James Lovelock, an independent British scientist, had measured CFC-11 in the lower atmosphere. Emphasizing that the compound posed "no conceivable environmental hazard," he viewed it as an indicator of the earth's wind and weather patterns. His estimates of atmospheric concentrations for CFC-11 roughly matched industry figures on cumulative global production, indicating that the chemical was apparently imperishable (Lovelock 1973:194–96).
Two chemists at the University of California at Irvine, F. Sherwood Rowland and Mario Molina, argued that although CFCs are insoluble and chemically inert in the troposphere, "odd chlorine" atoms in the stratosphere could initiate a catalytic chain reaction with ozone molecules (Molina and Sherwood 1974). Their paper defined the parameters of the ensuing controversy, the most important of which, from a policy perspective, was the issue of time scale. Because the atmospheric lifetimes of CFCs are 40 to 150 years, a steady-state concentration would not be reached for decades, even if CFC production ceased immediately. Rowland and Molina were particularly disturbed by the rapid growth in CFC production, which had doubled roughly every five years since World War II. On the basis of 1973 growth rates, they predicted that ozone would be depleted between 7 and 13 percent before stabilizing by the end of the next century. They called for an immediate ban on aerosol propellants, which accounted for half of all CFC usage in the United States.
While the public was shocked by the Rowland-Molina hypothesis, 5 the scientific infrastructure and the federal bureaucracy were well prepared to take up the issue as a result of CIAP and related studies. In the words of Carroll Bastian, who cochaired the federal task force charged with coordinating the scientific assessment, the response to the new CFC-ozone problem was "an unusual (if not unique) example of federal interagency coordination on an environmental regulatory question" (1972:167). The federal study affirmed the Rowland-Molina hypothesis but postponed its policy recommendations until the National Academy of Sciences completed its study (Interagency Task Force on Inadvertent Modification of the Stratosphere 1975). Some states, such as Oregon and New York, were unwilling to wait and passed legislation restricting the use of CFCs as aerosol propellants. These states had access to the same science as did the federal government and other states; they simply chose to err on the side of caution.
Industry called for more research. The international Manufacturing Chemists Association (now the CMA) increased funding for its Fluorocarbon Program Panel (FPP), established in 1972 shortly after Lovelock measured CFCs in the atmosphere. Most scientists I interviewed believe that the FPP did not censor the work it funded, but they also observed that its early grants were for projects that might undermine the Rowland-Molina hypothesis.
Du Pont, the world's largest CFC producer and maker of half of all CFCs in the USA, proclaimed that if studies showed "that chlorofluorocarbons cannot be used without a threat to health, Du Pont will stop production of these compounds" (United States Congress 1974). (As the scientific evidence mounted, Du Pont was frequently reminded of its pledge.) By tying its policy to the effects of CFCs on human health, Du Pont helped frame the issue in a way that focused debate on the health effects of ozone depletion rather than climatic and environmental change. While this mode of framing the issue was probably unintentional, its ramifications were felt throughout the policy-making process. Early on, the problem was framed almost entirely in terms of skin cancer.
Rowland and Molina soon discovered that the photochemistry was more complicated than they had originally believed. They ascertained that chlorine and nitric oxides could combine in the stratosphere to create chlorine nitrate (ClNO3), thereby forming "reservoirs" that would retard the ozone depletion rate by both chlorine and nitric oxides (Rowland and Molina 1976). They concluded that previous estimates of ozone destruction were too high, leading some to accuse the scientists of alarmism (Dotto and Schiff 1978:255). The new discovery prompted the National Academy of Sciences to postpone the release of its final report for several months.
The report, reflecting the new data, cut the estimate of ozone depletion in half, from 14 percent in an early draft to 7 percent. Even so, the report's policy recommendations to defer a regulatory decision seemed flimsy in light of its conclusion that continued releases of CFCs at 1973 levels could reduce ozone in the upper stratosphere by as much as 50 percent (National Research Council 1976). Rowland later lamented that the report "established a debilitating precedent at a crucial time in the whole affair when [it] advocated a delay in regulation" (Brodeur 1986:80). Industry, of course, claimed that the report vindicated its own position.
It is worthwhile to consider the scientific merit of the main arguments used by detractors of the ozone depletion theory in the early years, for these persisted throughout the controversy. 6 The least sophisticated argument was that, because ozone varies greatly under natural conditions, a decrease of several percentage points globally would not be disastrous. The fallacy in this logic is seen clearly by analogy with temperature. While a ten-degree change in one place on any given day is of no great concern, a decrease of ten degrees averaged globally would be catastrophic.
Industry also seized upon reports that ozone had increased over the Northern Hemisphere during the 1960s to undermine the Rowland-Molina hypothesis. There are three problems with this line of reasoning. First, many scientists attributed the increase in ozone to the ban on atmospheric testing of nuclear weapons early in the decade. Second, the increases might have been greater without CFCs during that period. Third, and most notably, industry's reasoning ignored the fact that the effects of CFCs are delayed because of their long atmospheric lifetimes. This point later became immaterial as satellite data indicated declining ozone levels in the 1970s and 1980s.
A third fallacy is the exaggeration of the "self-healing effect." As ozone is destroyed in the upper atmosphere, more ultraviolet light enters the lower atmosphere, where it is absorbed by diatomic oxygen molecules to make more ozone. But each ozone molecule destroyed in the upper stratosphere is not matched by a new one further down. Besides, the computer models had already incorporated the self-healing process, which is why they had predicted so much more depletion at the poles: the self-healing effect drops off as the intensity of ultraviolet radiation decreases with latitude. Furthermore, even if sufficient replacement ozone were produced, there would be no guarantee that it would rise up to exactly those spots where the layer had been weakened. Moreover, redistributing ozone would alter stratospheric temperatures, possibly precipitating dramatic climate changes. With the discovery in 1975 that CFCs are extremely potent greenhouse gases, the link between ozone depletion and climate change became increasingly salient (Ramanathan 1975).
Despite the academy's indecisiveness in its 1976 report, Russell Peterson, chairman of the President's Council on Environmental Quality declared that "we cannot afford to give chemicals the same constitutional rights that we enjoy under the law; chemicals are not innocent until proven guilty" (Brodeur 1986:74). Considering that he had been a Du Pont chemist for over twenty years, his request that federal agencies develop plans to regulate CFCs was remarkable. Peterson's voice was added to an emerging environmental policy discourse premised on what has come to be known as the "precautionary principle," i.e., that, in the face of scientific uncertainty, regulators should act to prevent harm rather than wait until damage occurs (Bodansky 1991).
In its amendments to the Clean Air Act in 1977, Congress called upon the EPA administrator to regulate any substance "which in his judgment may be reasonably anticipated to affect the stratosphere," thereby mandating preventive action based on the best scientific knowledge available. 7 The fact that empirical evidence of ozone destruction need not precede regulatory action later became an important factor in formulating the U.S. position during the international negotiations.
The 1977 legislation also required the United States to try to convince other nations to adopt regulations mirroring its own. Only Canada, Sweden, and Norway, all of which were heavily influenced by the 1976 academy report, followed the U.S. lead in implementing an aerosol ban (Stoel 1983:59). Despite pressure from the USA, the European Economic Community (EC) refused to adopt an aerosol ban. The British and the French were most resistant and remained so during the later negotiations (Jachtenfuchs 1990). The lack of success abroad was one reason that the EPA's proposed reductions in nonaerosol uses of CFCs never went beyond a notice in the Federal Register. Other factors included the lack of readily available substitutes and diminished public interest following the aerosol ban.
Despite industry's protests that no substitutes were available, the day after the aerosol phaseout was announced, Robert Abplanalp, inventor of the original aerosol spray valve and a vocal critic of the Rowland-Molina hypothesis, unveiled a new propellant to replace CFCs (Roan 1989:85). The hydrocarbon substitutes were used in the United States after 1978 but were not adopted abroad on a large scale until after the Montreal Protocol went into force.
The events leading to the aerosol ban set a pattern that continued throughout the ozone controversy: industry questioned the science and claimed that no replacements for the risky chemicals were available, but once the regulations were in place, substitutes quickly came on the market. The availability of substitutes, then, has typically been more contingent on the dominant policy discourse, translated into market signals, than on any scientific or technical factors.
Although the CFC-ozone controversy receded into the background politically during the late 1970s and early 1980s, it continued to receive substantial scientific attention. The state of knowledge paralleled the evolution of computerized atmospheric models. Over 192 chemical reactions and 48 photochemical processes are involved in ozone depletion caused by CFCs, but no models reflect all of them (Brasseur 1987:7). The first models in the mid-1970s were one-dimensional, averaging local effects of a single perturbation to yield a uniform global picture (National Research Council 1976:323–31). Later in the decade, two-dimensional models were developed to take into account latitudinal distributions. These models predicted large losses of ozone in the upper stratosphere, calling attention to the possibility of dramatic climate change attributable to ozone depletion. Most modeling since the late 1970s has used two-dimensional models. Three-dimensional models, developed in the early 1980s, factored in wind turbulence and divided the earth into grids. Although they are dynamically more accurate, three-dimensional models are expensive to run and their chemistry is somewhat simplistic, precluding widespread reliance on them (interview with Robert Watson). The models were increasingly refined, and their results tended to converge; disparities in their predictions were more often due to new data rather than differences among the models.
Not only did the models improve, but reaction rates were being revised. In 1977, two researchers found that nitrogen oxides and hydroxides would react forty times faster than previously believed (testimony by Dr. Rowland; U.S. Senate 1987b:5–6). This implied that nitrogen would be inactivated more quickly, thereby decreasing the amount available to form reservoirs of chlorine nitrate. Thus, ozone could be depleted much faster than either Rowland and Molina or the National Academy of Sciences had predicted in 1976.
The 1979 NAS report, taking these findings into account, painted a bleak picture, predicting as much as 16.5 percent depletion by the end of the next century. It also included much more information on climate change and the potential for increases in skin cancer, disruption of the aquatic food web, and crop damage. The report concluded with a sense of urgency, declaring that CFCs should be regulated beyond the aerosol ban already in place (National Research Council 1979).
In the same year, Britain's Department of the Environment released a much more equivocal study (U.K. Department of the Environment 1979). While confirming the 16 percent depletion estimate, this report vacillated by emphasizing that the ozone depletion theory was still a mere hypothesis. It concluded by calling for more research before taking action. (One of the British scientists later admitted that the U.S. report was probably more accurate because it used better models [Roan 1989:99].) Not surprisingly, industry on both sides of the Atlantic stressed the inconsistencies between the studies and endorsed the latter's call for further inquiry (Brodeur 1986:76).
Industry also emphasized that ozone losses had not yet been measured. Du Pont responded to the 1979 studies by pointing out that no ozone depletion had ever been detected and that the depletion figures were merely computer projections based on a series of uncertain assumptions. A vocal minority of scientists, including Rowland and Molina, countered that verifying predictions of future events is inherently impossible. Using the logic of the precautionary principle, they argued, once depletion could be confirmed empirically, it would be too late to reverse the damage. Furthermore, most scientists believed that validating the ozone depletion hypothesis did not require detecting actual ozone losses; finding chlorine radicals (chlorine and chlorine oxide) would be sufficient.
Measurement of changes in ozone, in parts per trillion, presented an array of problems. A few ground-based monitoring instruments, called Dobson stations, had been in place since the 1920s, with many more added after the International Geophysical Year in 1957. Their data, however, was considered unreliable: the stations were not well calibrated with one another, and they are not dispersed homogeneously, there being only a few stations in the Southern Hemisphere and almost none in the tropics. Prior to the Montreal Protocol, about a third of the stations did not report regularly to the World Ozone Data Center in Toronto (UNEP/WG.69/3:5). The stations were also vulnerable to interference from aerosols spewed into the atmosphere by volcanic eruptions. Further, CFC-caused ozone losses could not be distinguished from natural fluctuations, especially those associated with the eleven-year solar cycle. (As sunspot activity decreases, less ultraviolet radiation is emitted, resulting in a drop in ozone.) Finally, while the Dobson stations measured the total ozone column overhead, they could not decipher changes in distribution. Vertical profiles are measured by means of the indirect ground-based Umkehr method, which deduces levels from the differential absorption of light at various angles, and by balloon ozone-scondes, which take measurements at different altitudes. The former method is highly inaccurate because it is susceptible to disruption from aerosol particles; the latter is more accurate but very expensive (interview with Nien Dak Sze).
Until recently, ozone measurements were also gathered by the total ozone mapping spectrometer (TOMS) on the Nimbus 7 satellite, launched in 1978 to replace the Nimbus 4. Satellite data have the advantage of being able to differentiate ozone levels at various altitudes. Because computer models all predicted that the upper stratosphere will be effected most dramatically, this is important. But the satellite monitoring system is also vulnerable to confusion caused by aerosols like volcanic dust, and its instruments were deteriorating with age (Brasseur 1987:39).
Consequently, those who sought empirical proof on either side of the controversy were frustrated by the data itself. In 1981, Donald Heath of NASA's Goddard Space Flight Center, the principal analyst of the satellite data, compared data from the two satellites and believed he found a 1 percent decrease in total ozone during the 1970s. This finding, the first evidence of global ozone depletion, concurred with predictions of computer models. However, Heath's paper was rejected by the editors of Science because of questions about the accuracy of comparing data from two different satellites. That same year, the CMA released a summary of data from the ground-based Dobson network. Their analysis indicated that ozone levels had actually increased during the 1970s. Yet a National Oceanic and Atmospheric Administration (NOAA) study found that ozone over North America had decreased by 1 percent between 1961 and 1980 (Brodeur 1986:78). By the mid-1980s, the consensus was that ozone levels had remained essentially stable during the 1970s (Science Impact 1987).
While the scientists disputed the data, a bit of diplomatic progress was made. The EC Council of Ministers voted in March 1980 for all members to freeze their production capacity and reduce their consumption of CFCs in aerosols by at least 30 percent compared with 1976 levels (European Communities 1980). At an international meeting in Oslo the following month, delegates from all major CFC-producing nations agreed to reduce emissions from nonaerosol applications voluntarily. Delegates from the EPA went further by offering to freeze annual CFC production in the United States at 1979 levels.
However, changes in the political environment made further regulations in the USA appear unlikely. In the last days of the Carter administration, the EPA honored its pledge and published an Advance Notice of Proposed Rulemaking in the Federal Register outlining plans for future regulation of CFCs (EPA 1980). In response to this announcement, a coalition of five hundred CFC users and producers formed the Alliance for a Responsible CFC Policy to ensure that government did not regulate "based on unproven and unverified theory" (Alliance for a Responsible CFC Policy 1987:I-1). The alliance immediately organized an intensive lobbying effort to oppose the EPA's proposal. Then the promise of "regulatory relief" under the Reagan administration made it increasingly doubtful that the rulemaking would ensue. Anne Gorsuch Burford, the EPA's new administrator, made it clear that she did not take the threat to stratospheric ozone seriously (U.S. Senate 1981).
Seeing that further regulation was unlikely, Du Pont stopped its research on replacement compounds for CFCs. In the six years following the Rowland-Molina publication, Du Pont had spent over $15 million to develop commercially acceptable alternatives. Only a handful of compounds survived the tests, and no long-term toxicology studies had been done by 1980 (Du Pont 1980). 8 Clearly, the so-called essential CFCs would not be replaced as easily or as cheaply as the aerosols had been. In explaining Du Pont's suspension of its research program, Joe Steed, environmental manager for the Freon division, remarked, "There wasn't scientific or economic justification to proceed. How do you trade a possible [environmental] risk for a [business] risk that is real?" (Washington Post 1988b).
In 1981, a newly released international ozone trends study found no clear evidence of actual ozone loss but predicted that ozone would be depleted between 5 and 9 percent by the second half of the twenty-first century (WMO 1982). The new figures were based on refined chemical reaction rates and better data on the interactive effects of CFCs, carbon dioxide, nitrogen oxides, and methane. The NASA/WMO analysis also painted a more ominous picture of the biological and health effects than had past reports. A year later, the National Academy of Sciences published its third report, echoing much of the NASA/WMO analysis. Despite the bleak forecast, newspapers optimistically proclaimed that the danger was not as great as was previously believed. But this rosy picture only made sense in contrast to the 1979 prediction of 16.5 percent depletion.
A fourth academy report in 1984 seemed to offer even better grounds for complacency. Lowering its estimates for eventual ozone loss to 2 to 4 percent, the study relied on questionable economic data and some new chemical considerations. Ignoring the fact that CFC production was already rising as the world recovered from a recession, the report assumed that CFC output would remain stable. Methane, increasing by 1 percent annually, would also slow ozone depletion. Increasing concentrations of carbon dioxide and nitrous oxide might also counteract some of the negative effects of CFCs (National Research Council 1984).
However, though total ozone loss was expected to be low, the report substantiated earlier forecasts that the vertical distribution of ozone would be greatly perturbed. According to Rowland, this is important because "looking at the total ozone loss minimizes the importance of the issue. No one has yet succeeded in developing a scenario in which the increase of CFC's doesn't decrease upper stratospheric ozone" (quoted in Roan 1989:111).
In addition, while superficially heartening on ozone depletion, the report contained some disturbing news regarding climate change, claiming that the atmospheric concentration of CFCs was growing ten times as fast as carbon dioxide, the chief greenhouse gas. This information was alarming; each CFC molecule was thought to contribute as much to greenhouse warming as fifteen thousand carbon dioxide molecules (Rowland 1987). Combined with other trace gases, primarily methane and nitrous oxide, CFCs could contribute as much to global warming as carbon dioxide would. Moreover, ozone depletion from CFCs is mitigated primarily by rising methane levels, which also increase the risk of global warming. Thus, the 1984 NAS report demonstrated the inseparability of ozone and climate issues, though this was not the message that reached policymakers and the public (Thomas 1986; Science 1984).
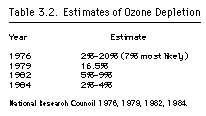
|
Interest among environmental groups was all but dead. With little support from his colleagues at the Natural Resources Defense Council (NRDC) and virtually no visible concern among other environmental organizations, Alan Miller decided to sue the EPA for neglecting to follow up on its Phase Two regulations. He was especially troubled by the fact that CFC output was increasing as the global recession subsided (interview with Alan Miller). Anne Gorsuch Burford had resigned as EPA head amid a storm of controversy in March 1983, providing an opening for a small group of proregulatory EPA staff (interviews with James Losey and Stephen Seidel). After giving William Ruckelshaus, the incoming EPA administrator, a grace period at his new post, Miller filed his suit in mid-1984. The agency persuaded Miller that a lawsuit compelling the USA to act unilaterally might undermine the sensitive international negotiations already under way. Miller agreed to delay the suit. But when it became clear that international regulatory measures were unlikely to be adopted at the diplomatic conference scheduled for March 1985 in Vienna, he filed the suit.
Two things happened at the EPA that would have an impact on the international negotiations. Ruckelshaus resolved a long-standing dispute between two EPA offices by transferring authority over stratospheric pollution from the Office of Toxic Substances, where it had been since the mid-seventies, to the Office of Air and Radiation. The Toxic Substances Office had supported Burford's position that international regulation of CFCs was premature, while officials in the Office of Air viewed CFCs as part of the greenhouse problem and supported international controls (interview with James Losey). The conflict between the two offices was resolved by the switch, with the newly combined office supporting the EPA's Office on International Activities, which felt that the United States should at least back a worldwide aerosol ban. Despite some objections from Burford allies within the State Department, the EPA succeeded in making its plan the formal U.S. policy.
The other important development was the appointment of Lee Thomas as EPA administrator in January 1985. Thomas, head of the agency's highly visible Superfund/Resource Conservation and Recovery Act programs under Ruckelshaus, was handpicked for the job by his predecessor. Initially, observers doubted that a nonlawyer could operate effectively in such heavily legalistic position. Others questioned the ability of a career bureaucrat to exercise political leverage within an administration whose ideological leanings ran counter to environmental regulation (Environmental Forum 1985:23–26). But Thomas, with an educational background in psychology, was also known for his management skills and his ability to work well with politicians. Soon after assuming his new post, Thomas was briefed on the ozone issue by scientists from NASA and NOAA. In his words, "I just took a black-and-white view when I saw the data. I knew we had to get [CFCs] out of process. It didn't appear that even a little bit of them was going to be safe" (interview with Lee Thomas).
The Road to the Vienna Convention
As the science appeared to be softening, the international meetings preparing for an ozone treaty helped to keep scientists' attention on the issue (interviews with Robert Watson and Ivar Isaksen). The United Nations Environment Programme, responding to a statement prepared by the World Meteorological Organization (1976:59), convened a meeting of scientists in 1977 to draft the World Plan of Action on the Ozone Layer (UNEP 1978:190). Despite its grandiose title, the plan simply called for research on ozone depletion and its effects and identified various UN organizations as lead agencies for specific research efforts. The primary international organizations working with UNEP on the World Plan of Action were the World Meteorological Organization (WMO) and the World Health Organization (WHO). The Coordinating Committee on the Ozone Layer (CCOL) was created to make periodic scientific assessments of the problem (UNEP/WG.7/25/Rev.1). According to R. S. Mikhail, a meteorologist and deputy director of UNEP's Environmental Assessment Division, which was overseeing ozone layer activities, UNEP did not intend to push for regulation of ozone-depleting substances. Rather, it saw its role as facilitating an international scientific consensus (Stoel, Miller, and Milroy 1980:276).
UNEP's role as scientific coordinator was surprising at the time, for its staff was small, geographically isolated, and not highly specialized in the relevant sciences. Moreover, its work up until that point was primarily in developing countries, whereas the ozone problem was centered in the developed world, both economically and scientifically. In fact, some observers saw the Organization for Economic Cooperation and Development (OECD) as the logical candidate for international leadership, since the major CFC producers were all OECD members (Stoel 1983).
In 1982, at the request of several Scandinavian countries, UNEP convened the first meeting of the Ad Hoc Working Group of Legal and Technical Experts to negotiate a framework convention on stratospheric ozone depletion. The UNEP secretariat prepared a paper for the working group in November 1982, in which it outlined alternative structures for protocols or annexes to a draft convention (UNEP/WG.78/3). The paper examined fifteen different conventions and protocols from its regional seas program. Following these examples, the group agreed to draw up a "framework convention" to be supplemented by protocols and annexes calling for specific control measures.
The negotiations became polarized when Finland and Sweden submitted a draft protocol, known as the Nordic Annex, calling for an aerosol ban and limits on other uses of CFCs. The EC, Japan, and the Soviet Union strongly opposed the proposal, arguing that the science did not mandate such measures. 9 The Europeans claimed that aerosol reductions and a production cap were sufficient. The United States also refused to back the Nordic Annex, arguing on procedural grounds that provisions for specific controls should not precede a framework convention. American supporters of the Nordic Annex maintained that the general U.S. objection to it sent the message that the U.S. aerosol ban had been a mistake. Eventually, with Burford's resignation, the United States joined the Nordics and Canada in what came to be known as the Toronto Group, after the group's first meeting in that city. Australia, Austria, and Switzerland were sympathetic to the Toronto Group's position (Sand 1985:42).
UNEP's working group met seven times between 1982 and 1985. It often seemed that no agreement would be reached among the fifty participating countries. The EC, bowing to the wishes of Britain and France, supported a cap on production capacity for CFC-11 and -12 and a 30 percent reduction of nonessential aerosol uses. The Toronto group viewed this proposal as self-serving; the EC had already adopted a 30 percent aerosol reduction, and its producers were only operating at about 65 percent capacity, the EC proposal would not require them to modify their behavior at all.
The Toronto group made four proposals in all, any of which would have reduced CFC emissions more than the EC proposal. But they too could be seen as self-serving because they focused on reductions in aerosol uses. The first two proposals were a ban or an 80 percent cutback on nonessential aerosol uses and exports. The third option was a 20 percent reduction of all CFCs within four years. The fourth was a 70 percent reduction of aerosol uses, accompanied by a production cap. The EC also argued that the Toronto Group's proposals did not take into account long-term growth of "essential" uses of CFCs, a conviction that the U.S. negotiators later came to share (interview with Robert Watson). Neither side was willing to accept new constraints on its own industries (Sand 1985:22).
Two legal issues hampered the negotiations: the procedure for settling interstate disputes and the voting status of the EC. After the 1984 World Court's verdict against the USA for mining Nicaragua's harbors, the United States reversed its position in support of compulsory referral to the International Court of Justice and instead insisted on a clause allowing the option of third-party mediation (article 11, Vienna Convention). On the second matter, the EC and other regional economic integration organizations would be permitted to vote on behalf of their states if those states chose not to vote (article 15).
In the end, the framework convention was adopted in March 1985 without any control provisions. Although no CFC controls were instituted, UNEP officials proudly proclaimed that the Vienna Convention was the first legal instrument to protect the global atmosphere. Mostafa Tolba, UNEP's executive director, declared it to be a sign of "political maturity" in that it dealt with a "distant threat," expressly recognizing an "intergenerational responsibility" (Sand 1985:20).
The Vienna Convention, like other treaties negotiated under UNEP's auspices, is careful to avoid the implication that the environmental norm it seeks to establish interferes in any way with the principle of state sovereignty. The second sentence of the preamble cites principle 21 of the 1972 Declaration of the United Nations Conference on the Human Environment, which provides that "states have . . . the sovereign right to exploit their own resources pursuant to their own environmental policies, and the responsibility to ensure that activities within their jurisdiction or control do not cause damage to the environment of other states."
In addition to establishing a general responsibility of states to protect the ozone layer, the Vienna Convention calls for various forms of scientific and technical cooperation among its parties. Despite the vagueness of some of these provisions, they were crucial for negotiating the Montreal Protocol. As early as 1982, it was recognized that the paramount need was for accurate global production statistics for all potential ozone-depleting substances (UNEP/WG.78/6:3). Without good production data, reliable predictions of ozone depletion cannot be made, regardless of the computer models' sophistication. Western Europe, the United States, and Japan report their production data for CFC-11 and -12 to the CMA. Communist countries, however, do not, and they refused to divulge them until after the Vienna Convention was adopted. In many countries, there was no legislation under which the government could compel firms to provide production statistics, especially when a company could show that disclosure would be prejudicial (UNEP/WG.78/6:5). Additionally, the CMA does not compile data on CFC-113 or the halons, so those data were subject to greater uncertainty. A major contribution of the Vienna Convention was that it prompted the gathering and disclosure of critical economic and technical information. In order to ensure some degree of commercial and national secrecy, the convention contains safeguards such as aggregation of data.
The convention also calls for scientific cooperation in developing computer models, monitoring the stratosphere, and developing alternatives to CFCs. The most immediate tangible results of these provisions were greater efforts to ensure that instruments were consistently calibrated.
Most importantly, the Vienna Convention establishes a norm, both in terms of state behavior and the environment itself. It mandates that states have a general obligation to refrain from activities that are likely to modify the ozone layer. No change in the ozone layer is acceptable; the environmental norm is an unmodified ozone layer, and the international norm is behavior that sustains that environmental norm. These norms are fundamentally an expression of the precautionary principle; thus, while no CFC controls were agreed upon, the Vienna Convention was important in that it legitimized a regulatory discourse. Empirical evidence of ozone depletion was not required before states must modify the behavior of CFC producers within their borders. Rather, the probability that certain actions would be detrimental to ozone should be enough, although the responses of states remained purely voluntary. Since this probability can only be known through scientific models, policymakers must look to the scientists. Consensual scientific knowledge becomes the progenitor of state conduct. But, in the face of scientific uncertainty, even actions that are only likely to deplete the ozone layer are unacceptable by the Vienna Convention's standards. In the absence of consensual knowledge, the precautionary principle should guide policy.
All of the participants I interviewed agree that the greatest significance of the Vienna Convention was that it represented the first global consensus that there was indeed a problem. They also believe that the failure to adopt control provisions at Vienna was fortuitous, since any protocol adopted at that time would not have been as comprehensive as the Montreal Protocol that was adopted only two years later.
Note 1: For a more detailed history of CFCs and refrigeration, see Linden and Didion 1987 and Cowan 1964. Back.
Note 2: Du Pont devised the shorthand notation for CFCs. The digit on far right is the number of fluorine atoms; the second digit from the right is the number of hydrogen atoms plus one; the third digit from the right is the number of carbon atoms minus one. CFCl3, which contains one fluorine atom, zero hydrogen atoms, and one carbon atom, becomes CFC-011 and then CFC-11, and CF2Cl2, which has two fluorine atoms, no hydrogen atoms, and one carbon atom, becomes CFC-012 and then CFC-12 (interview with Joseph P. Glas). Back.
Note 3: For the purposes of table 3.1, communist countries include the Soviet Union, the nations of Eastern Europe, China, and the other communist Asian nations. Most of these only began to make their production data available in 1986, during a series of workshops sponsored by UNEP and the EPA. Back.
Note 4: In an important sense, the politics drove the science on this issue by defining the salient areas of research. While climate change was perceived as a distant threat, skin cancer from ozone depletion was perceived by the public as an immediate, direct, and universal danger (see Ohi 1985). Back.
Note 5: Public reaction was so fervent in the USA that Congress received more letters on the issue than on any other since the Vietnam War (Brodeur 1986:71). Back.
Note 6: These arguments are examined in Dotto and Schiff (1978:208–14). Back.
Note 7: For a summary of the EPA's regulatory authority under the Clean Air Act, see "Protection of Stratospheric Ozone: Proposed Rule" (1987b). Back.
Note 8: Of all the producers, Du Pont had the only major research program for CFC substitutes. Back.
Note 9: Those countries that had already legislated regulations—the USA, Canada, Sweden, and the Netherlands—had high levels of environmental consciousness. The strongest opponents of CFC controls, including Britain, France, and Japan, lagged behind on issues like toxic substances, leaded gasoline, and nuclear proliferation (Stoel 1983:68–69). Thus national CFC policies can be seen as reflecting the strength of environmentalist discourse in each country. Back.